Published: 4 November, 2022
Contents
Increased risk of neurodevelopmental disorders with topiramate
A recently published study in JAMA Neurology has identified an increased risk of neurodevelopmental disorders in children exposed in utero to topiramate. The study, which involved nearly 4.5 million mothers and children in Nordic countries, found a 2.5-fold increase in autism-spectrum disorder and a 3-fold increase in intellectual disability in children born to women taking topiramate compared to women with epilepsy who were not taking any anti-seizure medicines. The risk of neurodevelopmental disorders with topiramate was higher than with sodium valproate.
The results of this study suggest that topiramate poses a similar risk of neurodevelopmental adverse effects as sodium valproate (or potentially higher) and the same level of caution should therefore be applied. Until now, there has been a lack of data to fully assess the magnitude of risk with topiramate. The results of this study have triggered a safety review of topiramate in the United Kingdom.
In New Zealand, topiramate is indicated for epilepsy and migraine prophylaxis in adults. Key points for prescribing any anti-epileptic medicines to females of child-bearing potential: Read more
- Anti-epileptic medicines provide significant benefit to people with epilepsy, but they are associated with an increased risk of adverse effects during pregnancy; higher doses or concurrent use of multiple antiepileptic medicines increases risk further
- Women of child-bearing age should be made aware of the potential risks of anti-epileptic medicines, but also of the risk of seizures during pregnancy, i.e. if an appropriate anti-epileptic medicine is not taken
- Two forms of effective contraception should be used by women of child-bearing age who are taking an anti-epileptic medicine; N.B. Some hormonal contraceptives interact with enzyme-inducing anti-epileptic medicines
- Pregnancy should be planned so that an anti-epileptic medicine regimen with the lowest risk, while balancing treatment efficacy, can be put in place
- If an anti-epileptic medicine is being considered for a condition such as migraine prophylaxis or neuropathic pain, consider other suitable treatment options first in a woman of child-bearing age
The Nordic register-based study of antiepileptic drugs in pregnancy (SCAN-AED) is a population-based retrospective cohort study that analysed live-born infants in Nordic countries between 1996 and 2017. Among nearly 4.5 million mothers and children, the use of topiramate was associated with an adjusted hazard ratio of 2.8 (95% CI, 1.4 - 5.7) for autism spectrum disorder and 3.5 (95% CI, 1.4 - 8.6) for intellectual disability. Higher doses of topiramate were associated with an increased risk of neurodevelopmental disorders; the adjusted hazard ratios for doses < 100 mg/day was 1.7 (95% CI, 1.0 – 2.8) and ≥ 100 mg/day 2.9 (95% CI, 1.3 – 6.7), compared to children in the general population. The same study found adjusted hazard ratios of 2.4 (95% CI, 1.7 - 3.3) for autism spectrum disorder and 2.5 (95% CI, 1.7 - 3.7) for intellectual disability following prenatal exposure to sodium valproate.
Summary of results and key points:
- The study included 4,494,926 children from births in Denmark (1997 - 2017),
Finland (1996 - 2016), Iceland (2004 - 2017), Norway (2005 - 2017) and Sweden (2006 - 2017):
- 24,825 children (0.6%) were exposed to anti-epileptic medicines during gestation and of these, 16,170 were born to mothers with epilepsy
- Prenatal exposure to an anti-epileptic medicine was defined as the mother receiving a dispensing of at least one prescription for an anti-epileptic medicine from her last menstrual period until birth
- At least one diagnosis of childhood autism, atypical autism or Asperger syndrome was required to meet the criteria for autism spectrum disorder. Children diagnosed with mild, moderate, severe or profound intellectual disability were also identified.
- The eight-year cumulative incidence of autism spectrum disorder was: 1.5% in children whose mothers were diagnosed with epilepsy but were not exposed to anti-epileptic medicines, 4.3% in children of mothers taking topiramate and 2.7% in children of mothers taking valproate
- The eight-year cumulative incidence of intellectual disability was: 0.8% in children whose mothers were diagnosed with epilepsy but were not exposed to anti-epileptic medicines, 3.1% in children of mothers taking topiramate and 2.4% in children of mothers taking valproate
- No increase in risk of neurodevelopmental disorders was detected in children of mothers with epilepsy who were exposed to monotherapy with other anti-epileptic medicines
- Moderate increases in adjusted hazard ratios were reported for children exposed to clonazepam (1.4; 95% CI, 1.1 - 1.9), oxcarbazepine (1.5; 95% CI, 1.2 - 2.0) and carbamazepine (1.6; 95% CI, 1.3 - 1.9), when compared to children in the general population; a weak association (1.3; 95% CI, 1.1 - 1.5) was detected between lamotrigine exposure and neurodevelopmental disorders
- No association was found between gabapentin, pregabalin and levetiracetam and neurodevelopmental disorders
- Dual therapy with anti-epileptic medicines was associated with increased risk of neurodevelopmental disorders except for levetiracetam and lamotrigine
- The combination of levetiracetam and lamotrigine may be an option for pregnant women whose epilepsy is not controlled with low dose monotherapy; more studies are required
Bjørk M-H, Zoega H, Leinonen MK, et al. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol (2022). https://jamanetwork.com/journals/jamaneurology/fullarticle/2793003?utm_campaign=articlePDF&utm_medium=articlePDFlink&utm_source=articlePDF&utm_content=jamaneurol.2022.1269
For further reading, also see: “Balancing the benefits and risks of prescribing antiepileptic medicines in women.” Bpacnz. https://bpac.org.nz/2018/antiepileptic.aspx
Thank you to Dr Peter Bergin, Neurologist, Auckland, for bringing this study to our attention and sharing resources on this topic.

The seventh annual #MedSafetyWeek is being held from 7th – 13th November 2022. Medicines regulators from 81 countries (and their stakeholders) are coming together for a social media campaign to encourage the reporting of suspected adverse effects of medicines. Look out for our posts!
This year the focus is on health professionals, patients and families/caregivers who report suspected adverse effects and how this contributes to medicines safety.
In New Zealand, suspected adverse effects to medicines and vaccines can be reported to the Centre for Adverse Reactions Monitoring (CARM) or directly via your practice management software.
Update on abortion services in New Zealand
Medicines for early medical abortion now available in the community
As of 1 November, the medicines used for early medical abortion (mifepristone and misoprostol) are available on prescription for dispensing in community pharmacies and on PSO. They may be prescribed by any relevant prescriber.
The New Zealand College of Sexual and Reproductive Health (NZCSRH), in conjunction with the Ministry of Health, has developed theory training in early medical abortion, first trimester vacuum aspiration surgical abortion and point-of-care ultrasound. The development of these training modules has been supported by bpacnz and they will be hosted on the bpacnz website.
A webinar is to be held on 16th November at 7pm, to provide health professionals further information about the first-trimester abortion training modules. To register your interest, click here.
For further information about the NZCSRH training modules, click here.
N.B. A training course for pharmacists about the safe and sensitive dispensing of medicines for early medical abortion is available from the Goodfellow Unit.
DECIDE abortion telehealth service fully up and running
On 1 November, the final phase of the DECIDE abortion telehealth service rollout was completed. As reported in Bulletin 44, DECIDE is a free nationwide initiative aiming to improve access to sexual health practitioner advice and, if required, access to early medical abortion for people within the first ten weeks of pregnancy.
New ACE inhibitor available in December: ramipril
Ramipril (Tryzan), an angiotensin-converting enzyme (ACE) inhibitor, will be funded from 1 December, 2022, without restrictions. Ramipril is indicated for people with hypertension, heart failure, progressive kidney disease and for the prevention of cardiovascular events in people with heart disease. Ramipril will be available in 1.25 mg, 2.5 mg, 5 mg and 10 mg capsules (see dosing information on NZF).
The addition of another funded ACE inhibitor option will be useful for patients transitioning from cilazapril, which is expected to be delisted in mid-2023.
For further information about prescribing ACE inhibitors, see: https://bpac.org.nz/2021/ace.aspx
N.B. This article will be updated with ramipril prescribing information by 1 December.
Proposal to widen access to meningococcal B vaccine
Pharmac is seeking feedback on a proposal to add meningococcal B vaccine (Bexsero) to the childhood immunisation schedule for infants aged up to 12 months, and for people aged 13 to 25 years entering into their first year of close living (specific criteria listed). Submissions are due by 8th November.
Read more
Meningococcal disease is the term used to describe the two types of illness caused by the bacterium Neisseria meningitidis: meningococcal meningitis and meningococcal septicaemia. There are multiple serotypes of N. meningitidis in New Zealand and the pattern of disease caused by each variant changes over time and by geographical area. The latest monthly surveillance report from ESR shows that between 1 January and 30 September, 2022 there have been 54 cases of invasive meningococcal disease, including two deaths. Almost half (48%) of the cases were in Māori and Pacific children aged under five years. Of the cases in which the variant was identified (44 cases), 82% were group B, 11% were group Y and 7% group W.
None of the currently available vaccines for meningococcal disease cover all of the variants. Bexsero is a recombinant vaccine that is broadly protective against group B disease. Conjugated vaccines are also available in New Zealand that protect against groups A, C, W and Y (Menactra, Nimenrix) or group C (NeisVac-C). For best protection, separate vaccinations that cover all groups are recommended. Vaccines are currently funded for high-risk individuals and close contacts.
It is proposed that from March 2023, children aged under 12 months will be able to receive three doses of the meningococcal B vaccine as part of the regular childhood immunisation schedule (with a catch-up programme available). People aged 13 to 25 years who are entering their first year of a close-living situation, e.g. boarding school hostels, tertiary education halls of residence, military barracks or prisons, will be eligible to receive two doses (with a catch-up programme for those already living in these situations).
N.B. This consultation also includes a proposal to continue to fund zoster vaccine (Shingrix) for the prevention of shingles. The initial supply of Shingrix was short-term only – the proposal also includes details about widening access to some groups.
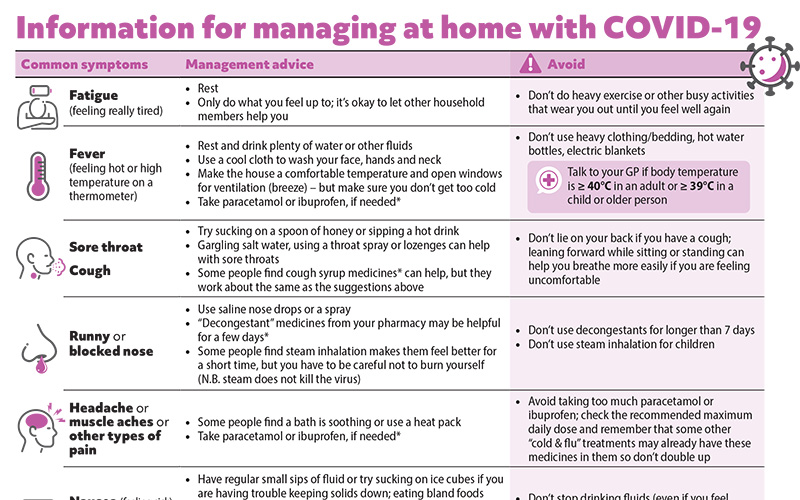
COVID-19 information sheet update
With COVID-19 cases numbers starting to rise again, we have updated our patient information sheet on managing at home with COVID-19. This can be downloaded and emailed or printed, or a link sent via text message for the patient or caregivers. Thank you to Dr Kevin Gabriel for getting in touch to request this update and helping us to “road test” the new version.
In Brief: Updates from IMAC
- You can now contact the Immunisation Advisory Centre (IMAC) with questions about vaccinations via the HealthLink tab on your practice management system; for immediate enquiries call 0800 IMMUNE (0800 466 863)
- IMAC is hosting webinars on PCV13 (15th November) and monkeypox vaccination (TBC); see website for details
To sign up for emails from IMAC on the latest news on immunisation, click here.
NZF updates for November
Significant changes to the NZF in the November, 2022, release include:
You can also read about any significant changes to the NZFC, here.
Coming soon: Your essential guide to liver function tests
Liver function tests (LFTs) are among the most commonly requested laboratory investigations in primary care. However, as with all other laboratory tests, it is essential that clinicians consider whether LFTs are being requested for the right patient at the right time, and have a clear understanding of how results will be interpreted based on the specific clinical context.
A comprehensive resource on LFTs, along with a spotlight on the key causes of liver disease and a B-QuiCK summary, will be available soon on our website. Keep an eye on your emails for early access.
Paper of the Week: What’s the best age to quit smoking?
As New Zealand works it way towards it’s Smokefree 2025 goal, we may need to come up with some new ways to support the hardest to reach people to stop. It is reported that as of 2020/21 around 9% of people aged over 15 years in New Zealand are daily smokers; this is half the rate reported in 2006/07. Only 1% of 15-17 year-olds and 8% of 18-24 year-olds smoke, compared to 14% and 25% in 2006/07. Smoking rates remain highest among Māori women (24%), but have also reduced considerably.
Providing education about the harms of smoking, and health benefits of quitting, is an important tool in helping motivate people to quit. A recently published study in JAMA Network Open that included over 550,000 adults in the United States found that smokers who quit prior to age 35 years are able to achieve a complete “reversal of risk”. The risk of death associated with smoking was reduced by 90% in those who quit before age 45 years and 66% in those who quit between age 45 to 64 years.
Therefore, to answer the title question, any age before 35 years is the “magic number”. You can tell patients that it’s never too late to quit, but the sooner they do, the more they have to gain.
Read more
- This was a prospective cohort study, using data from the US National Health Interview Survey, collected from 551,388 people aged 25 to 84 years, between 1997 and 2018
- The main outcomes were all-cause mortality, and mortality from cancer, cardiovascular disease and lower respiratory disease
- Never smokers were those who had smoked fewer than 100 cigarettes in their lifetime and ever smokers were those who had smoked more than 100 cigarettes; ever smokers who reported quitting within the five years prior to death were classified as current smokers, as were those who smoked every day or some days; ever smokers who quit prior to recruitment were classified as former smokers
- Compared with never smokers, current smokers had almost three times the all-cause mortality rate (Risk Ratio [RR]: 2.80)
- People who quit smoking had reduced mortality rates compared to those who continued: the RR compared to never smokers for those who quit smoking before age 35 was 1.03 (i.e. almost reversal of risk)
- Quitting smoking before age 45 years was associated with an approximate 90% reduction in the risk of excess mortality associated with continued smoking, and quitting between age 45 to 64 years was associated with an approximate 66% reduction in risk, irrespective of race and ethnicity
- On the assumption that the associations are causal, the researchers concluded that more than 40% of the deaths among ever smokers and more than 60% of deaths among current smokers were attributable to smoking, including 70% of deaths from cancer, 60% of deaths from cardiovascular disease and more than 90% of respiratory-related deaths
Thomson B, Emberson J, Lacey B, et al. Association between smoking, smoking cessation and mortality by race, ethnicity and sex among US adults. JAMA Netw Open 2022;5(10):e2231480. doi:10.1001/jamanetworkopen.2022.31480
 This Bulletin is supported by the South Link Education Trust
This Bulletin is supported by the South Link Education Trust
If you have any information you would like us to add to our next bulletin, please email:
[email protected]
 ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
© This resource is the subject of copyright which is owned by bpacnz. You may access it, but you may not reproduce it or any part of it except in the limited situations described in the terms of use on our website.





