Published: 23 June, 2023
Contents
New article – Long COVID: an evolving enigma

Preventing SARS-CoV-2 infection and managing the acute phase of illness has been the central focus of our healthcare system since the beginning of the COVID-19 pandemic. While most patients are expected to recover from COVID-19 within two to four weeks, some continue to experience symptoms 12 weeks or more post infection; this is defined as long COVID.
Given the diverse clinical picture associated with long COVID, it is not a “natural fit” for any one medical specialty, and primary care is often tasked with leading the management of affected patients. To help support clinicians, we have recently published a new long COVID resource, including advice to guide a progressive and tailored diagnostic work-up, and management tips to address the diverse range of possible symptoms.
Care plans should consider and address the wider consequences of ongoing symptoms, including effect on quality of life, emotional distress and psychological implications for the patient and their family/whānau. Not adequately managing these factors can impede and prolong recovery.
Read the full article here
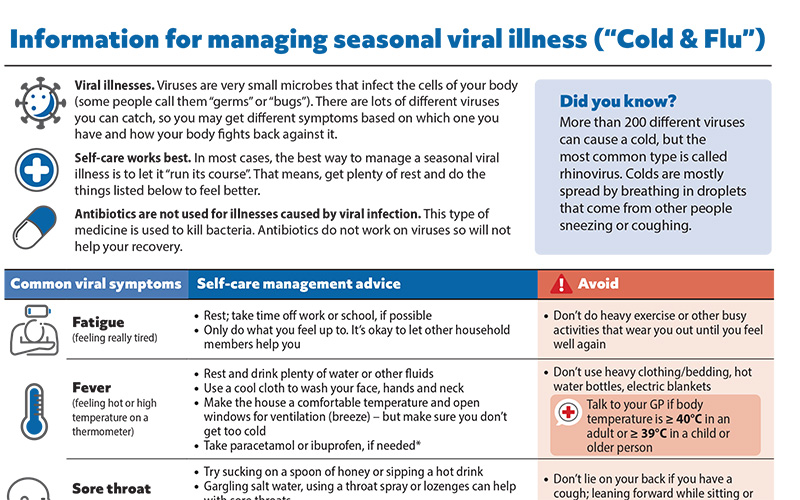
Patient information on managing seasonal viral illness
Helping patients cope with seasonal viral illness, i.e. “cold and flu” is an inevitable part of general practice, particularly during the winter months. To help support primary care health professionals in providing advice, we have developed a patient resource on managing seasonal viral illness at home. This information sheet can be downloaded and emailed or printed, or a link sent via text message, for the patient or caregivers.
We have also recently updated our patient resource on managing COVID-19 at home
Candesartan with hydrochlorothiazide funded from 1 July
Pharmac has announced that candesartan cilexetil with hydrochlorothiazide tablets will be fully funded for patients with hypertension, from 1 July, 2023. As previously mentioned in Bulletin 75, this medicine will be funded without restriction. This decision gives prescribers a funded fixed-dose combination treatment following the recall and delisting of quinapril with hydrochlorothiazide (Accuretic; see Bulletins 53 and 61).
Two tablet strengths will be funded*: candesartan cilexetil 16 mg with hydrochlorothiazide 12.5 mg and candesartan cilexetil 32 mg with hydrochlorothiazide 12.5 mg.
*Candesartan cilexetil 32 mg with hydrochlorothiazide 25 mg is also available but will not be funded
For further information on the management of hypertension in primary care, see: bpac.org.nz/2023/hypertension.aspx
Folic acid in wheat-based flours
From mid-August, all non-organic bread-making wheat flour either produced or sold in New Zealand must be fortified with folic acid. Organic and non-wheat based flours are exempt. The transition process to fortified flour began in 2021. Folic acid fortification of bread aims to reduce the incidence of neural tube defects, and is a way to ensure that women of childbearing potential have some level of folate, should they become pregnant.
Folic acid supplementation for women planning a pregnancy, or who are pregnant, is still necessary even if folic acid fortified bread is consumed. The recommended dose is 800 micrograms daily for at least four weeks prior to conception and for the first trimester. A 5 mg folic acid tablet daily is recommended for women at high risk, e.g. personal or family history of a neural tube defect, taking anti-epileptic medicines, with diabetes, who are obese or with malabsorption, e.g. due to coeliac disease.
N.B. The 800 microgram tablet of the currently funded brand of folic acid is not gluten free, however, the currently funded 5 mg tablet is gluten free and should be prescribed to women who cannot tolerate or consume gluten.
For further guidance on folate and folic acid for reducing neural tube defects, see: https://www.tewhatuora.govt.nz/for-the-health-sector/health-sector-guidance/folate-and-folic-acid/
Anyone for flu vaccine?
There are still many eligible adults and children who have not received their funded influenza vaccination. The national target is for at least 75% of adults aged 65 years or older to be vaccinated this flu season. As of 16 June, the overall vaccination uptake in this group is 61%, but rates are lower in Māori (53%), Pacific (50%) and Asian peoples (52%).
Māori and Pacific peoples aged 55 years and older are also eligible for funded influenza vaccination this year, however, the current level of uptake in people aged 55 to 64 years is only 27% for Māori and 30% for Pacific peoples.
Funded access has also been widened this year to include children aged six months to 12 years. Overall vaccination uptake in this group is currently 8%; higher in children of Asian ethnicity (16%), and lower in Māori (3%) and Pacific (6%) children.
Manatū Hauora, Ministry of Health, publishes data on uptake of vaccinations by district, updated weekly; check out how your area is doing here.
Encourage influenza vaccination opportunistically during routine appointments and ensure patients who meet eligibility criteria for funded vaccination are aware that they can receive a flu vaccine for free.
New Zealand-based online CBT course for health anxiety
Just a Thought is a New Zealand organisation that offers free online cognitive behavioural therapy (CBT) courses and hosts other resources for a range of mental health conditions. A new online CBT course has now been released for people with health anxiety.
The course uses CBT that has been specifically designed for this condition, and provides people with the necessary knowledge, skills and strategies to overcome anxiety associated with their health. The course can either be completed by the patient in a self-guided manner or through prescription by a clinician.
View all the courses available by Just a Thought here
Safe Access to Opioids: Engagement summary released
In March, 2023, Manatū Hauora, Ministry of Health, asked for public feedback regarding proposed approaches to the regulation of opioid prescribing in New Zealand. A summary of the feedback has now been published and will be considered when advising the Minister of Health on further amendments to the regulation of opioid prescribing. bpacnz and the New Zealand Formulary (NZF) provided a joint submission.
The full report can be found here
Read more
In December, 2022, Manatū Hauora, Ministry of Health, released amendments to the Misuse of Drugs Act relating to the prescription of controlled drugs in New Zealand. This included extending the amount of a Class B controlled drugs that can be prescribed at once using an electronic prescription (through the New Zealand ePrescription Service). After these amendments came into force, several concerns were raised regarding whether established controls to reduce opioid-related harms were still appropriate. Following a review of current controls, the following proposals were submitted for public feedback:
- Option 1: No regulatory change
- Option 2: Strengthen guidance to encourage good prescribing practices
- Option 3: Strengthen guidance and change regulations* to:
- reduce the prescribing limit for Class B opioids to one month (for both electronic and physical prescriptions) with an exemption for prescribing opioids for patients with cancer and those in palliative care
- require a peer-review process for repeat opioid prescriptions for patients with non-cancer pain
- ensure appropriate prescribing limits within the regulations for all prescribers of controlled drugs, including opioids
- insert a ten-day, or similar, dispensing restriction
*It was noted that if the specific regulatory changes proposed in option three were found to not be appropriate, they would not be progressed
Public feedback to the proposals argued that current controls for opioid regulation would no longer be appropriate given the recent regulatory changes and there was potential for an increase in opioid-related harms. For example, patients with opioid dependence may be able to access larger quantities of opioids and the removal of prescription limits could make it harder to identify inappropriate prescribing. Further regulatory amendments may be required to find the right balance between reducing the risk of opioid-related harms while maintaining access for patients that require these medicines.
For further information on the state of opioid prescribing in New Zealand, see: https://bpac.org.nz/2022/opioids.aspx
Pharmac seeking feedback on funding biosimilar trastuzumab
Pharmac has released a proposal to award Principal Supply Status to Herzuma (a biosimilar form of IV trastuzumab - Herceptin) from 1 December, 2023. This would result in Herzuma becoming the main funded brand of trastuzumab available in New Zealand. The proposal also includes widening access of trastuzumab to people with locally advanced or metastatic HER2 positive gastric cancer who meet Special Authority criteria. Proposed changes to the funding of trastuzumab will allow any relevant practitioner to apply for Special Authority (including renewal applications). Submissions are due by 30 June, 2023.
Read more
Trastuzumab is biological medicine indicated and currently funded for HER2 positive breast cancer. Herceptin is the currently funded brand.
If the proposal is accepted, Herzuma would be listed on the Pharmaceutical Schedule from 1 December, 2023 and from 1 June, 2024, it would have Principal Supply Status. Patients starting treatment with trastuzumab would be dispensed the biosimilar*, Herzuma rather than Herceptin, the currently funded medicine from 1 December, 2023.
*Biosimilar medicines are not identical to reference medicines (in this case, the currently funded brand). This is due to the complexities of manufacturing biological medicines from living organisms. Biosimilars are expected to have the same efficacy and safety as the reference medicine.
Changes to Special Authority criteria would be made so that people currently prescribed Herceptin will be able to continue taking it until it is delisted from the Pharmaceutical Schedule in June, 2024. People who need to change back to Herceptin for clinical reasons, e.g. adverse event after taking Herzuma, will be able to access funded Herceptin through the Exceptional Circumstances Framework.
Also, from 1 December, 2023, people currently taking IV trastuzumab (either Herzuma or Herceptin) who have responded positively to treatment can opt for a “treatment holiday”. After a discussion with their prescriber, people can choose to pause IV trastuzumab treatment with the aim to increase their quality of life. If disease progression is detected while on a “treatment holiday”, trastuzumab (Herzuma) would be reinitiated.
For further information on biosimilars, see: https://bpac.org.nz/2020/biosimilars.aspx
Paper of the Week: Assessing children with short stature in primary care
The height of a child largely depends on genetics, hormones and nutrition. Nutrition is the predominant factor that determines height during the first two to three years of life, after which, growth hormone and thyroid hormone are the main determinants until puberty. During puberty, height is determined by growth hormone, oestrogen and testosterone.
The cause of short stature in children is most often familial. In some cases, it may be due to a constitutional delay of growth and puberty, which is initially managed with watchful waiting. However, for other children, short stature (or reduced rate of growth) may indicate an underlying health issue such as chronic illness, lack of adequate nutrition or psychosocial issues. Short stature is more prevalent in females and in children living in low socioeconomic areas.
Early detection and intervention are important to improve clinical outcomes. A 2023 article published in the British Journal of General Practice discusses practical recommendations for the assessment of a child presenting with short stature. They note that this is often overlooked in girls and children from ethnic minority groups, as it may be assumed that their short stature is not abnormal.
Key points
- Consider further investigations into the possible causes of short stature in any child presenting with symptoms or signs of a chronic illness. In addition, further assessment is warranted in any child presenting to primary care with:
- A height less than the second percentile; or
- A height more than three percentile spaces below what is expected based on their parent’s height (mid-parental percentile); or
- Poor growth rate/height velocity (growth failure), i.e. a decrease in height of more than one percentile
- The cause of most children’s short stature is familial or constitutional delay of growth and puberty. Pathological causes of short stature (i.e. a growth disorder) are grouped into either primary or secondary:
- Primary – e.g. skeletal dysplasia, chromosomal disorders
- Secondary – e.g. inadequate nutrition intake, systemic disease or inflammation (e.g. Crohn’s disease), endocrine disorders, psychosocial issues
- The evaluation should begin with a targeted history, including ethnicity, gestation period and birth weight, neurocognitive development and behaviour, family history (including parental height, timing of puberty), medicines (e.g. corticosteroids), symptoms or signs of disease or a chronic illness (e.g. weight loss or reduced appetite, nausea, vomiting, fatigue, recurrent infections, headache, changes in vision) and any psychosocial issues, e.g. neglect, abuse.
- A physical examination should then be performed. This should include measuring height and plotting on a growth chart, as well as assessing weight and head circumference (in infants). Also examine for signs of hypothyroidism, for the presence of dysmorphic/disproportionate features (e.g. short arms or legs) and perform a cardiovascular examination (e.g. to exclude a heart murmur).
- Accurate measurement of length/height is essential. Poor technique or inaccurate interpretation has the potential for misdiagnosis.
- The length of children aged 0 – 2 years should be measured using a calibrated infant measuring device. From age two years, a manual or electronic wall-mounted stadiometer should be used to measure the child’s standing height. Gestational correction should be applied for those born < 37 weeks.
- A child’s potential height as an adult can be estimated based on a combination of their parent’s heights (i.e. mid-parental height; see the full article for calculation, or an online calculator is available here). This measurement can then be compared against the child’s current height percentile to determine whether they are growing as expected or whether they have familial short stature.
- If the child’s current height percentile is more than three percentile spaces below the mid-parental height percentile, consider further investigation (e.g. laboratory tests noted below), or referral to an appropriate specialist as required
- If indicated, laboratory tests may be requested to help exclude common growth disorders, e.g. full blood count, renal function tests, liver function tests, calcium, phosphate and alkaline phosphatase, thyroid function tests (TSH), “coeliac screen”
- Height velocity or growth rate can also be monitored over time if there are initial concerns about the child’s height; a decrease in height of more than one percentile may suggest abnormal growth and require further investigation
 Red flags requiring further investigation
Red flags requiring further investigation
- The presence of symptoms or signs of disease or a chronic illness, e.g. weight loss, anaemia, altered fat distribution, dysmorphic features or disproportion
- Further investigations should also be considered in children with short stature who present with multiple non-specific symptoms or signs, e.g. ongoing headaches, nausea, vomiting, diarrhoea or constipation
- No signs of puberty by age 13 years in females or age 14 years in males
- If the child’s height is < 0.4th percentile for age and sex or less than the second percentile for age and sex if other red flags are present
- If the child’s height percentile is more than three spaces below the mid-parental height percentile
- Decrease in height of more than one percentile
Storr HL, Freer J, Child J, et al. Assessment of childhood short stature: a GP guide. Br J Gen Pract 2023;73:184–6. doi:10.3399/bjgp23X732525
 This Bulletin is supported by the South Link Education Trust
This Bulletin is supported by the South Link Education Trust
If you have any information you would like us to add to our next bulletin, please email:
[email protected]
 ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
© This resource is the subject of copyright which is owned by bpacnz. You may access it, but you may not reproduce it or any part of it except in the limited situations described in the terms of use on our website.





