Published: 11 March, 2022
Contents
COVID Care app is here

As many of you would have seen, the Bpac Covid Care app is now live; we have had a great response so far. Bpac Covid Care is a decision support and management application integrated with MedTech Evolution and Medtech 32, that combines the most up to date guidance, clinical pathways and bestpractice decision support capability to help general practice safely triage and monitor patients with COVID-19 at home. It is provided to you free-of-charge by the South Link Education Trust and Bpac, supported by Medtech Global, as part of our ongoing commitment and service to general practice.
Covid Care makes managing your patients with COVID-19 easier by:
- Integrating relevant patient demographics, long term conditions and prescribed medicines from the PMS
- Using decision support to provide best practice recommendations based on the patient’s symptoms and signs
- Automatically generating recalls based on the patient’s risk categorisation within your Medtech Recalls Contact list
- Providing data within an individual PMS, that can then be used for claiming purposes
COVID Care is available now in your Medtech bestpractice dashboard. For further information about the app, including a link to an instructional video, see: https://bpac.org.nz/2022/covidcare.aspx
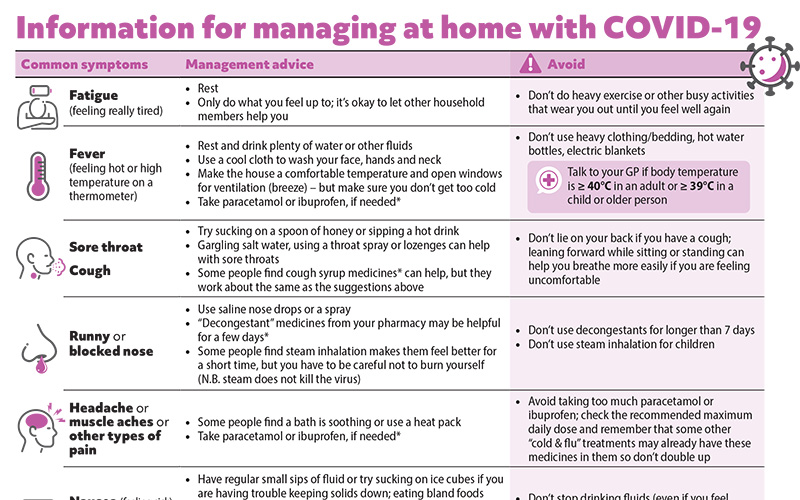
Patient information on managing COVID-19 at home
To support the COVID Care app, we have developed a patient resource on managing at home with COVID-19. This can be downloaded and emailed or printed, or a link sent via text message, for the patient or caregivers.
Third and fourth doses of COVID-19 vaccine for people with severe immunocompromise
Primary course: The COVID-19 Vaccine Technical Advisory Group recommend that people aged ≥ 12 years with severe immunocompromise receive a third primary dose of the Pfizer COVID-19 vaccine. The third primary dose should be administered at least eight weeks, but no more than three months, after the second dose*. If there are current or planned immunosuppressive treatments, the third dose should be delayed until two weeks after the period of immunosuppression, in addition to the period of clearance of the therapeutic agent.
Booster doses for ≥ 18s: A schedule of a primary course of three doses and a booster dose is recommended for people with severe immunocompromise aged ≥ 18 years. A booster dose is ideally given six months after the third primary dose but may be given from three months. Booster doses are not currently approved for use in people aged < 18 years.
N.B. The recommended regimen of three primary doses plus a booster (i.e. a series of four doses) is not approved, so either the third primary dose or the fourth dose (if the third primary dose is regarded at the time as a booster*) needs to be prescribed. If the third dose is entered into CIR as their booster, the fourth dose should be recorded as an additional vaccine in the primary/standard case schedule.
* If the third primary dose is given more than three months after the second dose, it has essentially become a booster dose, i.e. not part of the primary course. IMAC supports that a fourth dose can still be given three months later, ideally at six months.
For further information on third primary doses, see: https://covid.immune.org.nz/covid-19-vaccines-nz/getting-vaccinated/boosters-and-third-primary-dose-information
Novavax is now available
Novavax (Nuvaxovid), a recombinant protein vaccine against COVID-19, is now available in New Zealand for people aged 18 years and older who wish to use an alternative COVID-19 vaccine. The Pfizer COVID-19 vaccine remains the recommended vaccine for most people.
Read more
Novavax can be used as a primary course with two doses, spaced three weeks apart. A four-week interval between doses is recommended when used with a different COVID-19 vaccine. Novavax is not currently approved as a booster dose.
Novavax can be administered before, after or at the same time, as most of the other national scheduled vaccinations. Exceptions include, Zostavax where a seven-day interval is advised before or after administering Novavax, and the Shingrix and Fluad Quad vaccines where at least a three-day interval is recommended.
As international use of Novavax is limited, there is currently insufficient data on adverse effects and use during pregnancy. The Pfizer COVID-19 vaccine is the preferred vaccine for people who are trying to conceive, pregnant or breastfeeding.
Further information and resources, including a COVID-19 vaccine comparison chart, are available here.
Opioid consumer information leaflet
As part of an ongoing effort to minimise opioid misuse and dependence in New Zealand, Medsafe has published a consumer information leaflet on the risks associated with opioid medicines; available here to view, download and print.
In June, 2021, the Medicines Adverse Reaction Committee (MARC) recommended that Medsafe develop a consumer information leaflet to highlight the risks of opioid medicines following global concerns over opioid misuse and dependence. Read here to find out what other recommendations were made.
Brief update on influenza vaccines
The influenza vaccination programme is expected to commence from 1 April, 2022. PHARMAC has issued a proposal to extend funded vaccinations to include Māori and Pacific peoples aged between 55 and 64 years. The supplier of influenza vaccinations for 2022, Seqirus, has advised that the purchase cost to vaccinators will increase by $2 per dose; this cost will be fully reimbursed when vaccinating eligible people, however, the cost may need to be passed on to people who self-fund their vaccination.
IMAC has a dedicated website for health professionals to keep up to date with the latest information about the 2022 Influenza Immunisation Programme: www.influenza.org.nz
New Zealand Formulary updates for March
Significant changes to the NZF in the March, 2022, release include:
- Updated dosing of misoprostol to reflect use for medical termination of pregnancy
- New information on therapeutic drug monitoring has been added to the monograph for sodium valproate
- Severe Cutaneous Adverse Reactions (SCARs) has been added to the adverse effects of omeprazole
- Updated dosing regimen for bendroflumethiazide
PHARMAC medicine supply issues
The NZF contains up to date information about medicine supply issues. The following issues relating to medicine supply have recently been announced by PHARMAC
Pregabalin 25 mg capsules
PHARMAC has advised that there is limited supply of pregabalin 25 mg capsules due to global supply chain issues; 75 mg, 150 mg and 300 mg capsules are unaffected. Resupply is expected by early April, 2022, however, to extend the availability of current stock, stat dispensing has been removed and pharmacists should only dispense monthly lots until further notice. Other strengths can continue to be dispensed all-at-once.
Nutricia products
Nutricia has advised PHARMAC that there will be ongoing shortages of some special foods products throughout the first half of 2022 due to the global impact of COVID-19 on supply chains. Click here for information on affected products. Prescribers are advised to avoid indicating a specific brand or flavour on prescriptions to allow flexibility for pharmacists when dispensing.
Konsyl-D
PHARMAC has been advised of a supply issue with Konsyl-D. An alternative funded product, Macro Organic Psyllium Husk, is available for people who are currently taking Konsyl-D. This product is sourced from Australia and is classified as a food product, containing 100% psyllium husks, rather than a registered medicine like Konsyl-D. Pharmacists may need to assist patients with checking the packaging of Macro Organic Psyllium Husk for information on the recommended quantity to use.
Paper of the Week: Statin intolerance
Statin discontinuation due to intolerance is an important clinical challenge in primary care, and is associated with an increased risk of cardiovascular events. A meta-analysis recently published in the European Heart Journal (176 studies, including over four million patients), found that the overall prevalence of statin intolerance is low, and might often be over-estimated. The authors identified possible risk factors that influence the risk of statin intolerance.
Main findings
- The pooled prevalence of statin intolerance was 9.1% and lower when using international definitions, e.g. National Lipid Association (7%), International Lipid Expert Panel (6.7%), European Atherosclerosis Society (5.9%)
- The prevalence of statin intolerance when used for primary prevention in patients with familial hypercholesterolaemia, hypercholesterolaemia, dyslipidaemia and type 2 diabetes was 9%, 12%, 13% and 6%, respectively
- The prevalence of statin intolerance when used for secondary prevention in patients with stable coronary artery disease, acute coronary syndrome, myocardial infarction and stroke/transient ischaemic attack was 8%, 13%, 13% and 5.4%, respectively
- Older age, female sex, Asian and African-American ethnicity, obesity, type 2 diabetes, alcohol consumption, exercise, hypothyroidism and chronic liver and renal failure were associated with a higher risk of statin intolerance
- Concomitant use of antiarrhythmic medicines, calcium channel blockers and higher statin doses were also associated with a higher risk of statin intolerance
- The concept of statin intolerance is often overestimated leading to unnecessary statin discontinuation and suboptimal lipid levels
- Authors recommend that clinicians closely assess patients who present with possible statin intolerance symptoms, to reduce unnecessary discontinuation, and use the results of this meta-analysis to encourage adherence to statin treatment
Bytyçi, I, Penson, P, Mikhailidis, D, et al. Prevalence of statin intolerance: a meta analysis. European Heart Journal 2022; ehac015, https://doi.org/10.1093/eurheartj/ehac015
For further information on prescribing statins, including managing adverse effects, see: https://bpac.org.nz/2021/statins.aspx
 This Bulletin is supported by the South Link Education Trust
This Bulletin is supported by the South Link Education Trust
If you have any information you would like us to add to our next bulletin, please email:
[email protected]
 ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
© This resource is the subject of copyright which is owned by bpacnz. You may access it, but you may not reproduce it or any part of it except in the limited situations described in the terms of use on our website.





