Published: 31 March, 2022
Contents
New article – Diverticulitis: pockets of knowledge
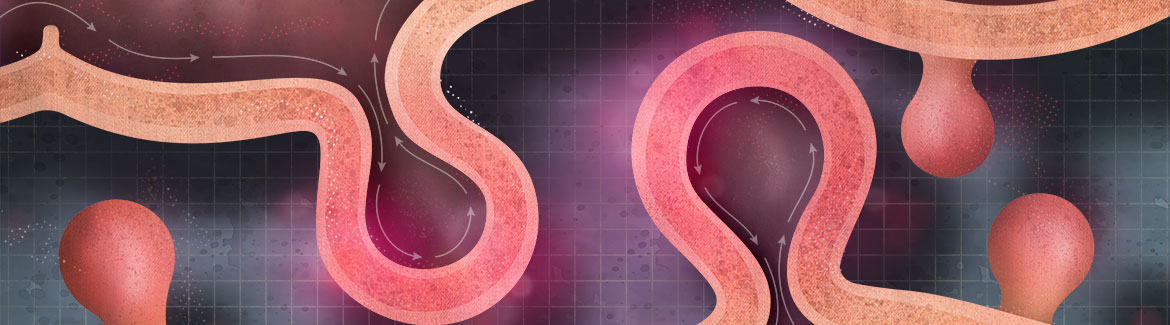
Diverticulitis occurs when small pockets in the wall of the large bowel become inflamed, usually without a specific identifiable cause. Conventionally, diverticulitis is treated with antibiotics based on the assumption of a bacterial aetiology. However, there has been a global shift to less intensive management based on evidence that inflammation has a more significant role than previously thought. This means that many patients can be treated in the community with analgesia and selective use of oral antibiotics.
Read the full article here.
 Got no time for that or just need your answers now? Click for B-QuiCK.
Got no time for that or just need your answers now? Click for B-QuiCK.
Clinical audit – Identifying patients who are not participating in regular cervical screening

A new clinical audit has been published on identifying patients who are not participating in regular cervical screening. The purpose of this audit is to initiate conversations about cervical screening with patients who are not regularly participating in the programme, to identify their reason(s) why and then, if possible, to try to resolve these issues.
Ultimately the decision to participate in cervical screening is made by the patient, therefore it is difficult to audit practice in the usual way; hence this audit slightly differs from traditional ones. You will not need to search for and access the patient’s clinical notes to complete this audit. Instead, it can be completed over time opportunistically during consultations for other reasons with eligible patients until the required number of patients has been reached.
For further information on cervical screening and cervical cancer, see: https://bpac.org.nz/2022/cervical-cancer.aspx
South Link Education Trust announced as new sponsor of GPCME conferences
The following message was provided by Conference Matters and South Link Education Trust
We are delighted to announce that South Link Education Trust will be sponsoring the upcoming Rotorua and South General Practice Conference and Medical Exhibition (GPCME) events.
As a not-for-profit organisation, South Link Education Trust is committed to supporting the ongoing professional development of healthcare professionals in New Zealand. We are proud to partner with GPCME to bring these important events to our community. Through this sponsorship, South Link Education Trust will be subsidising the registration fees for healthcare professionals, making it easier for them to attend and benefit from the valuable educational and networking opportunities that GPCME provides.
We would like to extend a special invitation to you to attend the Rotorua and South GPCME events. This is an excellent opportunity to gain valuable knowledge, skills and network with your peers. The event will feature a diverse range of speakers, workshops, and presentations, with topics ranging from clinical updates to practice management. We hope that you will join us for what promises to be two inspiring and informative events.
Details of the programmes are available at:
N.B. South Link Education Trust is the sole shareholder of bpacnz.
Syphilis case numbers increasing in New Zealand
Following a reported increase in syphilis cases in New Zealand, Manatū Hauora, Ministry of Health and the Institute of Environmental Science and Research (ESR) are raising awareness of this risk and recommending that people use prevention measures, particularly those who are pregnant and their partners. Data released by ESR, but not yet published, show a 41% increase in syphilis cases between the first (91 cases) and last quarter (140 cases) of 2022.
Read more
The majority of syphilis cases in New Zealand are among males. Men who have sex with men are at the greatest risk; in 2022, there was an 81% increase in syphilis cases in men who have sex with men between Jan/March to Oct/Dec. However, cases among heterosexual males are also increasing (along with cases in females). In 2022, there was a 76% increase in syphilis cases in heterosexual males between Jan/March to Oct/Dec. Congenital syphilis case numbers were also high in 2022, and disproportionately affected Māori and Pacific peoples. The full report with detailed 2022 data is expected to be published by ESR soon.
If left untreated, syphilis can cause significant damage to the nervous system, brain and other organs. In pregnancy, syphilis can cause congenital abnormalities, severe illness in infants, pre-term delivery and stillbirth. The early detection and treatment of syphilis in pregnancy can protect infants from infection. Syphilis testing is offered as part of the first antenatal blood tests, and it is recommended that all pregnant women be offered re-testing with their routine second antenatal blood tests.
Healthcare professionals can advise patients who engage in high-risk sexual activity to practice safe sex, including using a condom or oral dam, and having regular STI check-ups. Syphilis serology should be included as part of routine STI testing for all patients, and for anyone who is at increased risk of a STI or who presents with suspicious symptoms, e.g. genital, anal or mouth ulcer, generalised rash.
If syphilis is suspected, discussion with or referral to a sexual health physician is recommended to assist with interpreting serology results and determining an appropriate treatment regimen. N.B. Infectious syphilis is a notifiable disease.
For further information on syphilis, see: https://bpac.org.nz/2019/syphilis.aspx
Candida auris identified in New Zealand
Candida auris infection has been the subject of recent media attention due to the spread of antimicrobial resistant strains. Manatū Hauora, Ministry of Health has confirmed one case of C. auris has been identified in New Zealand (infection occurred overseas).
Read more
C. auris was first identified in Japan in 2009 and is developing into a significant problem globally. There are four strains of C. auris; South Asian, East Asian, African and South American, and there are reports of C. auris infections in over 30 countries. C. auris is of particular concern due to its resistance to common antifungal medicines. In the USA, nine in ten C. auris samples tested were resistant to at least one antifungal medicine and three in ten were resistant to two or more. C. auris can colonise the skin without causing active infection and conventional disinfectants (e.g. hydrogen peroxide) are not particularly effective against it.
Patients at increased risk of C. auris infection include those with implanted medical devices, e.g. central venous catheter, particularly if they have recently travelled overseas (ask about any time spent in a healthcare facility), have spent long periods of time in a healthcare facility, e.g. hospital or rest home, or have recently been treated with antibiotic or antifungal medicines.
Symptoms of C. auris infection vary but clinicians should be alert to patients with any of the above risk factors presenting with systemic symptoms such as persistent fever and chills. C. auris is most commonly associated with bloodstream, wound and ear infections.
For further information on Candida auris for health professionals, click here
Guidelines for healthcare workers on limiting infection and minimising contamination when inserting medical devices can be found here
New legislation about medicines that can impair driving
Medsafe has issued an Alert Communication following the introduction of new legislation* about medicines that can impair driving, as part of the prevention of drug driving. Aspects of this legislation that could impact patients include:
Read more
- The addition of Schedule 5, that lists 25 specific prescription medicines or illicit drugs identified as being the highest risk to road safety:
- Alprazolam, amphetamine, buprenorphine, clonazepam, cocaine, codeine, diazepam, dihydrocodeine, fentanyl, GHB, ketamine, lorazepam, MDMA, methadone, methamphetamine, midazolam, morphine, nitrazepam, oxazepam, oxycodone, temazepam, THC, tramadol, triazolam and zopiclone
- A new blood test that measures the blood concentration levels of the medicines/illicit drugs listed in Schedule 5. The blood concentration will determine the extent of the drug-driving offence.
If a driver tests positive for a Schedule 5 substance, a medical defence is available to them if they have a valid prescription for that medicine and were taking it as prescribed.
Healthcare professionals are advised to:
- Be aware of the prescription medicines listed in Schedule 5
- Check medicine data sheets (and NZF) when prescribing to determine the effect on driving
- Discuss with patients if any medicines they take could impair driving (including over-the-counter, pharmacist-only and prescription medicines)
- Ask patients whether they have any symptoms when taking their medicine(s) that could impair driving (and therefore they should be advised not to drive), e.g. dizziness, drowsiness, confusion, slurred speech, headache, nausea, blurred vision, slowed reaction times, inability to focus, mania
* Land Transport (Drug Driving) Amendment Act 2022, introduced 11 March, 2023.
Monitoring Communication update: abnormal uterine bleeding and anticoagulants
In August 2022, Medsafe asked prescribers to report any cases of abnormal uterine bleeding with oral anticoagulants: this was covered in Bulletin 58. The reporting period has now ended and the Centre for Adverse Reactions Monitoring (CARM) has received four reports of patients with abnormal uterine bleeding associated with rivaroxaban (between August 2022 and February 2023). There were no reports received for other oral anticoagulants. Bleeding and/or urogenital haemorrhage is listed as an adverse effect in oral anticoagulant data sheets. On balance, Medsafe advises that the benefit/risk for oral anticoagulants remains positive.
Prescribers should discuss this potential adverse effect with female patients prescribed anticoagulants, particularly rivaroxaban.
Reminder: Influenza season starts 1 April
The 2023 Influenza Immunisation Programme officially starts tomorrow (1 April). As reported in Bulletin 69, access to influenza vaccination has been widened this year to include children aged six months to 12 years, and Māori and Pacific peoples aged 55 – 64 years. Funded access remains for people aged > 65 years or with chronic health conditions. Full eligibility criteria can be found here.
Read more
The following quadrivalent flu vaccines are available for 2023:
- AFLURIA® QUAD JUNIOR: for use in children aged 6 – 35 months (i.e. < 3 years). Funded for children who meet eligibility criteria.
- AFLURIA® QUAD: for use in children aged three years and over and adults. Funded for children and adults who meet eligibility criteria.
- FLUAD® QUAD: for use in adults aged 65 years and over. Not funded.
- FLUQUADRI®: for use in children aged six months and over and adults. Not funded.
A new strain is included in this year’s influenza vaccines: A/Sydney/5/2021 (H1N1) pdm09-like virus. Other strains that are included remain the same as last year: A/Darwin/9/2021 (H3N2)-like virus, B/Austria/1359417/2021-like virus and B/Phuket/3073/2013-like virus.
The 2023 Flu kit booklet for healthcare professionals is now available. Stay up to date with the latest information and resources here.
NZF Patient Information Leaflets upgrade
Patient Information Leaflets in the New Zealand Formulary are being upgraded. The first change, an addition of a “Before you start” section, was made live on 17 March, 2023. Other changes will be made over time, including extended leaflets with some disease-specific information, the frequencies of adverse effects with pictorials and risk/benefit discussions.
Paper of the Week: The push to limit social media use
For many people, particularly young people, time spent using social media occupies a significant proportion of each day, and concerns are growing about the impact that this has on mental health. Many studies have linked frequent social media use with harm, including body image issues and eating disorders, but whether reducing the time spent on social media decreases these associated harms is largely unknown.
A 2023 study published in Psychology of Popular Media, a journal of the American Psychological Association, has examined the effects that reducing smartphone social media use has on mental health-related outcomes (appearance esteem and weight esteem) in younger people with emotional distress. A significant improvement in appearance esteem and weight esteem was found in participants who reduced social media use to one hour per day, compared to those with unrestricted access. Suggesting a reduction or limitation in social media use may, therefore, be an effective strategy when managing a patient with mental health concerns, particularly when body image is a factor.
Read more
- A randomised controlled trial was conducted over four weeks where 279 undergraduate psychology students (76% female, 23% male, 1% other) at a Canadian University were assigned to an intervention (social medial use limited to one hour/day) or control (no restriction on social media use) group. 220 participants were included in the final analysis.
- To be eligible to participate, students must have been aged between 17 and 25 years, regularly used social media on a smartphone (for at least two hours per day on average) and have symptoms of depression or anxiety. A younger demographic was chosen as they are likely to be at greater risk of experiencing the negative effects associated with social media use and body image issues.
- Participants were not told the purpose of the study. Social media use was monitored via a screen tracking programme and submitted daily during a one week baseline and a three week intervention period. To assess changes in appearance and weight esteem, measurements (using the abbreviated version of the Body Esteem Scale for Adults and Adolescents) were taken both at baseline and after the intervention, e.g. “I’m happy about the way I look” and “I am satisfied with my weight” on a 1 (never) to 5 (always) Likert scale.
- Using a device-based measure of social media use increases reliability and avoids recall bias
- For week one (baseline), all participants were told to use social media as usual. After the first week, participants randomly assigned to the intervention group were told to limit their social media use to one hour per day. The control group were to continue social media use as normal.
- Participants in the intervention group reduced social media use by approximately 50% from an average of 168 min/day during baseline to 78 min/day at the end of week four. The control group spent an average of 181 min/day using social media during baseline which increased slightly to 189 min/day at the end of the trial.
- Participants who limited their social media use to one hour/day, experienced significant improvements in their appearance esteem (from 2.95 to 3.15 points, P < 0.001) and weight esteem (from 3.16 to 3.32 points, P < 0.001). There were no significant differences for participants who used social media without restriction (i.e. control group). No significant sex differences were found (although the study was underpowered for this).
- Several limitations were noted including that:
- The intervention was for a short duration
- The study was limited to monitoring the use of social media on smartphones, but participants may have accessed social media using other devices, e.g. laptop, tablet
- The study population was students who volunteered to participate in a study where they had a 50% chance of being asked to limit their social media use, and so the results may not generalise to people who are less interested in reducing the amount of time spent using social media
Thai H, Davis CG, Mahboob W, et al. Reducing social media use improves appearance and weight esteem in youth with emotional distress. Psychol Pop Media 2023; [Epub ahead of print]. https://www.apa.org/pubs/journals/releases/ppm-ppm0000460.pdf
 This Bulletin is supported by the South Link Education Trust
This Bulletin is supported by the South Link Education Trust
If you have any information you would like us to add to our next bulletin, please email:
[email protected]
 ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
ASK A COLLEAGUE: Are they receiving these bulletins?
Sign up to our mailing list here
© This resource is the subject of copyright which is owned by bpacnz. You may access it, but you may not reproduce it or any part of it except in the limited situations described in the terms of use on our website.





