 View / Download pdf version of this article
View / Download pdf version of this article
Key practice points
- Patients with atrial fibrillation who have a very low risk of stroke are unlikely to benefit from antithrombotic treatment
- Aspirin monotherapy should not be prescribed solely for stroke prevention; anticoagulation is the preferred treatment
- On balance dabigatran appears at least as effective and may be safer than warfarin for the prevention of ischaemic
stroke and systemic embolism
- Dabigatran should NOT be prescribed to patients with valvular heart disease
The number of antithrombotic medicines is increasing, as is the challenge for general practitioners in advising patients
about treatment options and managing patients when antithrombotic medicines are initiated in secondary care, e.g. following
a percutaneous coronary intervention.
Antithrombotic treatment recommendations for patients with non-valvular atrial fibrillation depend on the patient’s
risk of ischaemic stroke which can vary by 20-fold depending on their age and clinical features.1
Managing stroke risk in patients with non-valvular atrial fibrillation
The individual stroke risk for patients with non-valvular atrial fibrillation needs to be assessed to determine if they
are likely to benefit from anticoagulant treatment.1
Previous guidance on the management of stroke risk has changed
Previously, all patients with atrial fibrillation were offered antithrombotic treatment and those with a CHA2DS2-VASc
score of 0 (see: “Atrial fibrillation management tools: CHA2DS2-VASc and
HAS-BLED”) were offered aspirin in preference to an anticoagulant. It is now recommended that these patients should
not be treated with either an anticoagulant or an antiplatelet at this time.2 Furthermore, aspirin monotherapy
should generally not be prescribed for the purpose of stroke prevention in any patients with atrial fibrillation: anticoagulation
is preferred.3
Guidance now recommends that anticoagulation be considered for all patients who have a CHA2DS2-VASc score ≥
1.4 Where it is uncertain if a patient will benefit from anticoagulant treatment discussion with a cardiologist
or neurologist may be beneficial.
Always consider the risk of bleeding before discussing anticoagulation treatment with a patient. This
risk, however, should not be overstated. Risk factors for bleeding in patients taking anticoagulant treatment include:4
- Increasing age
- Uncontrolled hypertension
- History of myocardial infarction, ischaemic heart disease or cerebrovascular disease
- Anaemia
- A history of bleeding
- The use of other medicines that increase bleeding risk, e.g. aspirin or other antiplatelet medicines and non-steroidal
anti-inflammatory drugs (NSAIDs)
There are a number of tools available that can be used to assess the risk of bleeding in patients with atrial fibrillation.
The HAS-BLED tool is used to identify modifiable risk factors that can be managed in patients undergoing anticoagulation
treatment.2
Warfarin or dabigatran to prevent thromboembolism?
If anticoagulant treatment is appropriate the decision needs to be made whether warfarin or dabigatran is the preferred
treatment option (Table 1). Patient preference plays a significant role in this decision.
On balance the evidence suggests that dabigatran, dosed appropriately, is at least as effective and may be safer
than warfarin for the prevention of ischaemic stroke and systemic embolism.
Dabigatran should NOT be prescribed to patients with valvular heart disease: patients with mechanical
heart valves who take dabigatran are at an increased risk of bleeding or experiencing a thromboembolic event compared
to what their risk would have been if they had been prescribed warfarin.8
Table 1: The advantages and disadvantages of dabigatran, compared with warfarin, for the treatment of patients with non-valvular atrial fibrillation
| The advantages of dabigatran |
The disadvantages of dabigatran |
- Superior stroke prevention with dabigatran 150 mg, twice daily
- Testing and dose adjustments are not currently required
- Onset of anticoagulation is rapid (two to three hours) compared with 48 – 72 hours with warfarin5
- Does not accumulate in the liver and safer in patients with hepatic dysfunction6
- Fewer interactions with other medicines and foods
- A reduced risk of intracranial haemorrhage with dabigatran 110 mg, twice daily
|
- An increased incidence of gastrointestinal adverse effects, e.g. dyspepsia
- Twice daily dosing required
- Caution required in patients with progressive chronic kidney disease (CKD)
- A small absolute increase in risk (0.27%) of acute coronary syndrome7
|
Ticagrelor is superior to clopidogrel in patients with acute coronary syndromes
It is increasingly likely that patients who have been diagnosed with an acute coronary syndrome will receive long-term
treatment with ticagrelor, twice daily, in preference to clopidogrel, once daily; both are used in combination with aspirin,
i.e. dual antiplatelet treatment. The choice of anticoagulant is usually made in hospital following diagnosis of an acute
coronary syndrome and treatment is then continued in the community for twelve months.
Unlike clopidogrel, ticagrelor is not a prodrug and therefore does not need to be processed by an enzyme (CYP2C19) to
be activated. This explains why ticagrelor is reported to produce faster, greater and more consistent inhibition of platelet
reactivity compared with clopidogrel.9 Due to ethnic differences in the prevalence of genetic polymorphisms
in the CYP2C19 enzyme that metabolises clopidogrel it has been suggested that Māori and Pacific patients should be preferentially
treated with ticagrelor over clopidogrel.10
How is ticagrelor initiated ?
Treatment with ticagrelor begins with 180 mg as a loading dose, then 90 mg, twice daily, for up to 12 months.5 Ticagrelor
should be taken in combination with low-dose aspirin.5 The most frequent adverse effect is a transient dyspnoea
that does not appear to be caused by bronchospasm; ticagrelor should be used cautiously in patients with asthma or COPD.5 Ticagrelor
should be discontinued five days before elective surgery.5 It is recommended that renal function be tested
within one month of initiation.5
Atrial fibrillation management tools: CHA2DS2-VASc and HAS-BLED
The CHA2DS2-VASc stroke risk assessment tool uses risk factors to calculate a score out of nine. This tool
is helpful for identifying patients at very low risk of stroke who may not benefit from treatment with an anticoagulant.2 Figure
1 shows the annual stroke risk for patients with a CHA2DS2-VASc score of 0 – 6.
Clinical feature |
Points |
Congestive heart failure |
1 |
Hypertension |
1 |
Age
|
1 or
2 |
Diabetes mellitus |
1 |
Stroke or transient ischaemic attack |
2 |
Vascular disease, e.g. peripheral artery disease, myocardial infarction, aortic plaque |
1 |
Female sex |
1 |
Total out of 9 = |
|
The HAS-BLED tool is used to identify modifiable risk factors in patients undergoing anticoagulation treatment.2 HAS-BLED
may also be useful in balancing the risks versus benefits of anticoagulation treatment in patients with atrial fibrillation
who have a CHA2DS2-VASc score of 1.2 HAS-BLED should not, however, be used to determine
whether a patient should be offered anticoagulation treatment as this decision should be based on stroke risk.2 A
HAS-BLED score > 2 is associated with a clinically significant risk of major bleeding.1
Risk factor |
Score |
Hypertension (systolic blood pressure > 160 mmHg) |
1 |
Abnormal renal and liver function |
1 point each |
Stroke (past history) |
1 |
Bleeding (previous history of bleeding or predisposition to bleeding) |
1 |
Labile INRs (unstable, high or insufficient time with therapeutic range) |
1 |
Elderly (aged over 65 years) |
1 |
Drugs or alcohol (including concomitant use of aspirin, other antiplatelet medicines and NSAIDs) |
1 point each |
Total out of 9 = |
|
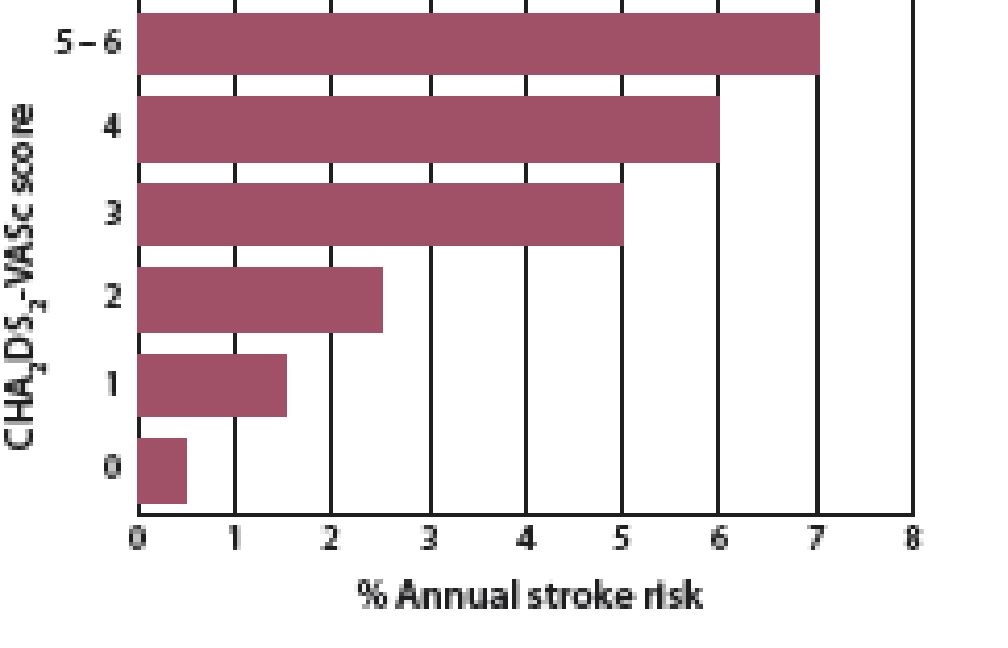
Figure 1: Annual stroke risk for patients with a CHA2DS2-VASc of 0 – 6
 For further information, see:
“An update on antithrombotic medicines – What does primary care need to know?” BPJ 67 (Apr, 2015).
For further information, see:
“An update on antithrombotic medicines – What does primary care need to know?” BPJ 67 (Apr, 2015).
References
- Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for
healthcare professionals from the American Heart Association/American Stroke Association. Stroke J Cereb
Circ 2014;45:3754–832. http://dx.doi.org/10.1161/STR.0000000000000046
- Hobbs FR, Taylor CJ, Jan Geersing G, et al. European Primary Care Cardiovascular Society (EPCCS) consensus guidance
on stroke prevention in atrial fibrillation (SPAF) in primary care. Eur J Prev Cardiol 2015;[Epub ahead of
print].
- National Institute for Health Care Excellence (NICE). Atrial fibrillation: the management of atrial fibrillation.
2014. Available from: www.nice.org.uk/guidance/cg180 (Accessed Dec, 2015).
- Scottish Intercollegiate Guidelines Network (SIGN). Prevention of stroke in patients with atrial fibrillation.
Available from: www.sign.ac.uk/pdf/AF_publication.pdf (Accessed Dec, 2015).
- New Zealand Formulary (NZF). NZF v42. 2015. Available from: www.nzf.org.nz (Accessed Dec, 2015).
- Weitz J. Antiplatelet, anticoagulant, and fibrinolytic drugs. In: Harrision’s principles of internal medicine.
McGraw Hill Medical 2012. pp. 988–1004.
- Uchino K, Hernadez A. Dabigatran association with higher risk of acute coronary events: meta-analysis of noninferiority
randomized controlled trials. Arch Intern Med 2012;172:397–402.
http://dx.doi.org/10.1001/archinternmed.2011.1666
- U.S. Food and Drug Administration. FDA Drug safety communication: Pradaxa (dabigtran etexilate mesylate) should
not be used in patients with mechanical prosthetic heart valves. 2012. Available from:
www.fda.gov/Drugs/DrugSafety/ucm332912.htm (Accessed Dec, 2015).
- Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes.
N Engl J Med 2009;361:1045–57. http://dx.doi.org/10.1056/NEJMoa0904327
- Larsen P, Johnston L, Holley A, et al. Prevalence and significance of CYP2C19*2 and CYP2C19*17 alleles in a New
Zealand acute coronary syndrome population: CYP2C19*2 in acute coronary syndromes. Intern Med J 2015;45:537–45.
http://dx.doi.org/10.1111/imj.12698