Part 1: Providing behavioural support for patients
Nicotine addiction is a disorder that should be considered at every patient contact. All patients who smoke should be encouraged to stop
and provided with cessation support. Patients who are not yet ready to quit smoking can be encouraged to reduce the amount they smoke and
provided with support in the same way as people who have committed to complete abstinence from tobacco. Patients trying to quit who view a
lapse in smoking abstinence as a hurdle, rather than a failure, are more likely to become permanently smokefree.
Treating the deadly habit
Half of people who take up tobacco smoking long-term, die from this cause.1 Approximately 5 000 people
in New Zealand die each year due to smoking related causes; 350 of these deaths are caused by second-hand smoke.2 People
who smoke cigarettes die ten years younger than non-smokers on average,1 an effect on mortality similar to
that of morbid obesity.3
The harm smoking causes can be undone
People who smoke can reverse the long-term effects of smoking if they stop early enough. Quitting smoking before age
40 years results in approximately nine more years of life expectancy compared to those who continue to smoke.1 Each
year of smoking beyond this age reportedly results in three months loss of life.3
Are we on track for a smokefree New Zealand?
In 2011, the New Zealand government committed to achieving a smokefree New Zealand by 2025. The aim is to reduce the
prevalence of smoking to less than 5% across all groups of people.4
The rate of smoking has been declining over New Zealand in the last decade. Current smoking (defined as smoking at least
once a month), was reported by 17% of the population sampled in 2013/14, a decrease from 20% in 2006/07.5 Encouragingly,
the rate of current smoking in people aged 15 – 17 years has dropped by half since 2006/07 and was 8% in 2013/14.5 However,
41% of Māori aged 18 years or over reported current smoking in 2013/14, unchanged from 2006/07.5 The growing
up in New Zealand study found that nearly 11% of pregnant women overall and over one-third of Māori women, smoke during
pregnancy.6 Adults living in the most deprived communities in New Zealand are 3.5 times more likely to smoke
than adults living in the least deprived areas once adjustments for age, ethnicity and sex have been made.5
Offer cessation support to every patient who smokes, at every contact
Nicotine addiction should be managed like other long-term health issues and be addressed at every patient contact, unless
it is inappropriate to do so.3 This can be challenging as patients may find it irritating if clinicians continually
point out the need to stop smoking. However, the majority of people who smoke wish that they did not. The 2009 New Zealand
Tobacco Use Survey found that 80% of current smokers aged 15–64 years would not smoke if they had their life over again.7 Initiating
discussions about smoking cessation in different ways is one approach to reducing repetition. For example, smoking cessation
conversations could begin by mentioning:
- Stoptober, a 31 day smokefree challenge that is run every October
- The idea of making the family home smokefree, starting from Christmas day
- The possibility of starting the new year with a quit attempt
- How quitting smoking improves cardiovascular risk
Tailor the ABC pathway to the individual patient
The risks of continued smoking and the benefits of quitting should be tailored to the individual in a non-judgemental
way. For example, the financial cost of smoking may be emphasised to some patients whereas others may find health reasons
more of a motivation. The revised ABC pathway for smoking cessation is:2
Ask about and document the smoking status of every patient.
Give Brief advice to stop to every patient who smokes.
Strongly encourage every person who smokes to use Cessation support and offer help accessing this. A combination
of behavioural support and smoking cessation medicine works best.
The offer of cessation support is particularly important in the ABC model. The authors of a systematic review estimated
that if all smokers were given advice to stop smoking, 25% would attempt to stop within six months of the consultation,
but this could be increased to 35% if advice was followed with an offer of cessation support.8 Without cessation
support half of all quit attempts fail within the first week.3
When cessation support is offered this is an opportunity to acknowledge and explore the barriers people have to being
smokefree. Health professionals who understand the day-to-day difficulties faced by people who smoke can point out ways
behavioural and pharmacological support helps people overcome their barriers to a healthier life.
What to do if a patient declines an offer of support
Document when a patient declines an offer of smoking cessation support and advise them that they will be offered cessation
support again at the next consultation.2 A New Ministry of Health target requires all patients who smoke
to have documented evidence of an offer of cessation support in the last 15 months (see: “Primary care achieves
health targets as new targets are announced”). This new target encourages more frequent use of the ABC model.
Varying the ways the pathway is implemented and discussed with patients may be necessary to maintain a fresh
approach.
 For further information see: “Smoking cessation beyond the ABC: Tailoring
strategies to high-risk groups”, BPJ 64 (Oct, 2014).
For further information see: “Smoking cessation beyond the ABC: Tailoring
strategies to high-risk groups”, BPJ 64 (Oct, 2014).
Cessation support can reduce smoking before a patient quits
People who smoke who are not yet ready to commit to a quit date are more likely to stop smoking if they are offered
cessation support.9 NRT can be prescribed to people who want to reduce the amount they smoke before they
quit (see: “A focus on pharmacological support”).2 There is evidence that quitting
smoking by reduction is as effective as quitting abruptly.10
A pragmatic approach would be to acknowledge the challenge the patient faces in becoming smokefree, offer them treatment
and encourage them to reduce their cigarette consumption by half. A follow-up consultation could review the patient’s
progress and offer referral to a support provider. Smoking cessation providers can contact people who are ambivalent about
quitting smoking to discuss their options.2
Table 1: Examples of smoking cessation support services available in New Zealand
Support provider |
Services available |
How to access |
Quitline |
Telephone counselling, text and online support.
The smoking cessation support service most often used in New Zealand. Once a person has been referred by the primary
care team they will be contacted by the service in one to three days.2 |
Phone 0800 778 778 or register at www.quit.org.nz
|
Aukati KaiPaipa |
Face-to-face coaching in individual and group settings.
A smoking cessation service based on a Māori health framework, operating from more than 30 sites throughout New Zealand. |
For further information, see: www.aukatikaipaipa.co.nz/contact
|
Pacific Quit Smoking Service |
Face-to-face coaching, telephone and text support |
Email: [email protected] |
Comprehensive care |
Mobile quit bus in Auckland area, face-to-face and telephone support. Interpreters may be available who speak
Samoan, Chinese (Mandarin and Cantonese), Korean, Hindi and Gujarati, Czech and Slovak |
For further information, see: www.comprehensivecare.co.nz
|
Innov8 smokefree |
Home visits in the Christchurch area for women who are pregnant, telephone and text support and biochemical confirmation
of abstinence |
For further information, see: www.innov8smokefree.co.nz
|
Behavioural support options available
Smoking cessation interventions can be divided into behavioural and pharmacological support. The benefits of these two
forms appears to be additive and behavioural support improves adherence to pharmacological treatment.3 The
two approaches are used in combination where possible. It is not known which is the most effective form of behavioural
support and each probably has a small additive effect; some patients may find different forms more effective than others.3 Interventions
with some evidence of improving smoking cessation treatment adherence include: education, positive feedback from a health
professional, reminders, psychological support and counselling.11
What appears to be common across successful methods is that they hold the “quitter” accountable and engender some form
of loyalty towards the person who is supporting the quit attempt.3
Refer all patients who want to quit to a cessation support provider
All patients who accept an offer of cessation support should be referred to a support provider, or be supported in primary
care.2 There are a number of different free smoking cessation support services available depending on the needs
of the patient. Quitline and Aukati KaiPaipa are national smoking cessation support providers. Some areas may have specialised
services depending on the demographics of the local population (Table 1). The primary care team should be familiar with
the local services available and can find out more information by contacting their local DHB.
Supporting patients who are attempting to become smokefree
Throughout a person’s quit attempt support from the primary care team is beneficial. This may involve education and
correcting mistaken beliefs that make it more difficult for people to stop smoking.
“Smoking does not reduce stress – in fact it creates it.”Many smokers claim that smoking improves their
mental health by alleviating emotional problems, stabilising mood and reducing stress, depression and anxiety.12 This
belief originates from their experience with nicotine. People who smoke often have withdrawal symptoms which they relieve
by smoking the next cigarette. Each cigarette that is smoked therefore contributes to the addiction and reinforces the
misbelief that cigarettes reduce stress, when in fact they cause it. People who believe that smoking is a coping mechanism
for stress often find it harder to quit smoking than people who smoke predominately for pleasure. A study of over 2 000
people using a smoking treatment service in England found that the one-year quit rate was 20% for people who smoked mainly
for pleasure, but 11% for people who smoked mainly as a coping strategy.13
“The withdrawal symptoms will pass – hang in there” People who are struggling with nicotine withdrawal
can be reassured that the symptoms largely pass after a few weeks.2 Sleep disturbances can be expected to last
for one week, poor concentration and urges to smoke due to nicotine withdrawal may last for two weeks.2 Irritability,
depression and restlessness are likely to resolve within four weeks.2 An increased appetite may last for more
than ten weeks.2 Patients may report an increase in cough and sputum production, although this is uncommon.14 Patients
who express concern about an increase in productive respiratory symptoms after quitting can be reassured of the health
benefits of being smokefree and that any increase in cough is likely to be transitory.
“You may put on a bit of weight – but you’ll be fitter and enjoy exercise more” Quitting smoking is
associated with an increase in bodyweight of 4 – 5 kg after 12 months of abstinence.15 However, this increase
varies and approximately 16% of people who quit smoking lose weight.15 The majority of weight gain occurs within
three months of quitting;15 reassure patients that weight gain is unlikely to continue. The negative consequences
of any weight gain can be balanced against the reduced risk of smoking–related illnesses and increased cardiovascular
fitness.
Behavioural support can increase treatment adherence and prevent smoking relapses
Smoking relapses, i.e. a return to regular smoking, are characterised by many intermittent lapses in abstinence over
days or weeks.16 How the person responds emotionally to these lapses is thought to determine whether they fully
relapse to smoking or if they are able to re-establish abstinence.16 Smoking relapse is thought to be more
likely, if following a lapse, a person blames themselves, feels excessive guilt and experiences a loss of self-efficacy;
collectively referred to as an abstinence violation effect.16 In other words, if a person interprets a lapse
in smoking as a lack in will-power they are more likely to lapse multiple times and start regular smoking again; perhaps
because abstinence is viewed as being pointless and smoking inevitable.16
If a person who is trying to quit reports a lapse the primary care team can help by attempting to maintain the patient’s
morale and improve their self-efficacy, i.e. their belief in their ability to quit smoking.16 This may include
focusing on the length of time that they have been able to stay smokefree and explaining that the odd lapse is normal.
The reasons for the lapse can be discussed as a learning opportunity and framed as a positive in the sense that a trigger
for smoking can be avoided in the future.

Primary care achieves health targets as new targets are announced
In the final quarter of 2014/15, primary care, for the first time, achieved documented evidence that more than 90% of
patients in New Zealand who smoke and were seen by a health practitioner were given brief advice and support to quit smoking.17 Across
all DHBs, 90.5% of people who smoked were offered brief advice and support to quit smoking; a 1.4% increase from the previous
quarter.17 The Southern DHB and MidCentral DHB still have some way to go to achieving the target with 74%
and 82% respectively of patients having documented evidence of the ABC model being used.17 Northland, Hawke’s
Bay, Hutt Valley, Capital and Coast, Taranaki, Canterbury and Wairarapa DHBs are slightly below the 90% threshold.17
The Ministry of Health has now released a new target for primary care. This is for 90% of PHO-enrolled patients who
smoke to have been offered help to stop smoking in the last 15 months, even if they have not attended a primary care clinic.18 Patients
who smoke who do not regularly attend general practice may need to be contacted and followed-up by phone calls or letters
to achieve the new target.
 For further information see:
www.health.govt.nz/new-zealand-health-system/health-targets/about-health-targets/health-targets-better-help-smokers-quit
For further information see:
www.health.govt.nz/new-zealand-health-system/health-targets/about-health-targets/health-targets-better-help-smokers-quit
Part two: A focus on pharmacological support
All patients who want to quit smoking should be offered pharmacological cessation support. Nicotine replacement therapy (NRT) is often the first smoking
cessation medicine people try and is also recommended for use by people who want to reduce the amount that they smoke. Most people trying to quit smoking do
not use enough NRT and the use of multiple forms, e.g. patches and gum or lozenges, is highly effective relative to other treatments. Bupropion and nortriptyline
are fully subsidised smoking cessation medicines that have approximately the same efficacy as treatment with one form of NRT. Varenicline is subsidised with
Special Authority approval for people who have been unsuccessful in quitting with other cessation medicines; it has approximately
the same efficacy as treatment with combination NRT.
Addiction and nicotine replacement therapy: fighting fire with fire
Smoking is the most reinforcing and dependence-producing form of nicotine administration.19 Nicotine reaches
the brain 10–20 seconds after an inhalation of tobacco smoke; faster than could occur with intravenous administration.19 The
speed with which nicotine from tobacco smoke enters the blood stream and crosses the blood brain barrier allows smokers
to titrate their levels of nicotine by altering the number of inhalations, the volume of each inhalation and the length
of time they hold the smoke in their lungs.19
Pharmacological support reduces the urge to smoke when people are experiencing nicotine withdrawal. It also enables
the person to unlearn the perceived association between smoking and reward over several months, after the withdrawal symptoms
have abated.3
Nicotine replacement therapy is often the first cessation medicine used
The patient’s preferences, likely adherence to treatment as well as their previous experience of smoking cessation aids
and the possibility of adverse effects are all important considerations when recommending smoking cessation medicines.
Nicotine, as replacement therapy, is usually the first-line smoking cessation medicine recommended. Using NRT approximately
doubles a person’s chances of quitting smoking.20 The different forms of NRT are thought to be equally effective.20 If
a person is not ready to quit, NRT can be used to reduce the amount that they smoke before they stop.2
Multiple forms of NRT are recommended
Combination NRT is recommended for people who smoke more than ten cigarettes a day or who smoke within one hour of waking;21 combination
NRT may be as effective as varenicline.20
Nicotine from NRT is absorbed more slowly than from tobacco smoke; nicotine from patches takes one hour to be detected
in the blood.19 New Zealand guidelines recommend the concurrent use of both long-acting NRT, e.g. patches,
and short-acting NRT, e.g. gum or lozenges, to enable sustained slow-release nicotine delivery and more rapid delivery
of nicotine when people are experiencing a craving, e.g. when friends are smoking.2 Combination NRT treatment
is more effective than NRT monotherapy, i.e. patches and gum are more effective than patches alone.20 Nicotine
mouth spray (1 mg nicotine per spray) is licensed for use in New Zealand, but is not currently subsidised on the Pharmaceutical
Schedule.21 Because people using NRT that is not inhaled do not get the same “rush” from nicotine as when
they are smoking, NRT is considered to have a low potential for misuse.19 Dependence on nicotine gum is estimated
to occur in 2% of users.22
How much NRT should be prescribed?
Most people who are trying to quit smoking do not use enough NRT.21 To determine an appropriate regimen
the time until first cigarette after waking is combined with the total number of cigarettes a person smokes each day (Figure
1). People who are severely dependent on nicotine may benefit from wearing two nicotine patches.
NRT is typically prescribed for 8 – 12 weeks but people may take it for longer to prevent a relapse in smoking.21 The
strength of nicotine patches can be slowly reduced over the patient’s course of treatment (see NZF for details).23
Managing the adverse effects of NRT treatment
All forms of NRT may cause palpitations.23 Oral NRT can cause irritation of the throat, dry mouth or increased
salivation, and gastrointestinal symptoms are common although these are most likely due to swallowed nicotine.23 Mild
skin reactions often occur with the use of nicotine patches.21 Nicotine patches can be removed overnight
if sleep is disturbed.21 If a patient reports feeling nauseous with the use of NRT, reduce the dose. Data
on the safety of the long-term use of NRT is scarce. In vitro studies provide limited evidence that nicotine
could theoretically accelerate cancer formation in people who used to smoke.24 However, it is broadly accepted
that if a person is at a high risk of relapse the long-term use of NRT should be encouraged rather than risk a return
to smoking.21, 24
Preventing relapses in patients taking nicotine replacement therapy (NRT)
Patients who report a smoking lapse while taking NRT should be urged to continue treatment. A study of over 300 people
who had smoked at least 15 cigarettes, daily, for a minimum of five years randomised participants wanting to quit smoking
to placebo or nicotine patches.16 Overall, patients using NRT had smokefree periods between smoking lapses
that were nearly twice as long as patients using placebo patches, and were significantly less likely to relapse to regular
smoking.16 The protective effect of NRT against smoking relapse diminished with the number of lapses and
there appeared to be little benefit in NRT treatment after eight lapses.16
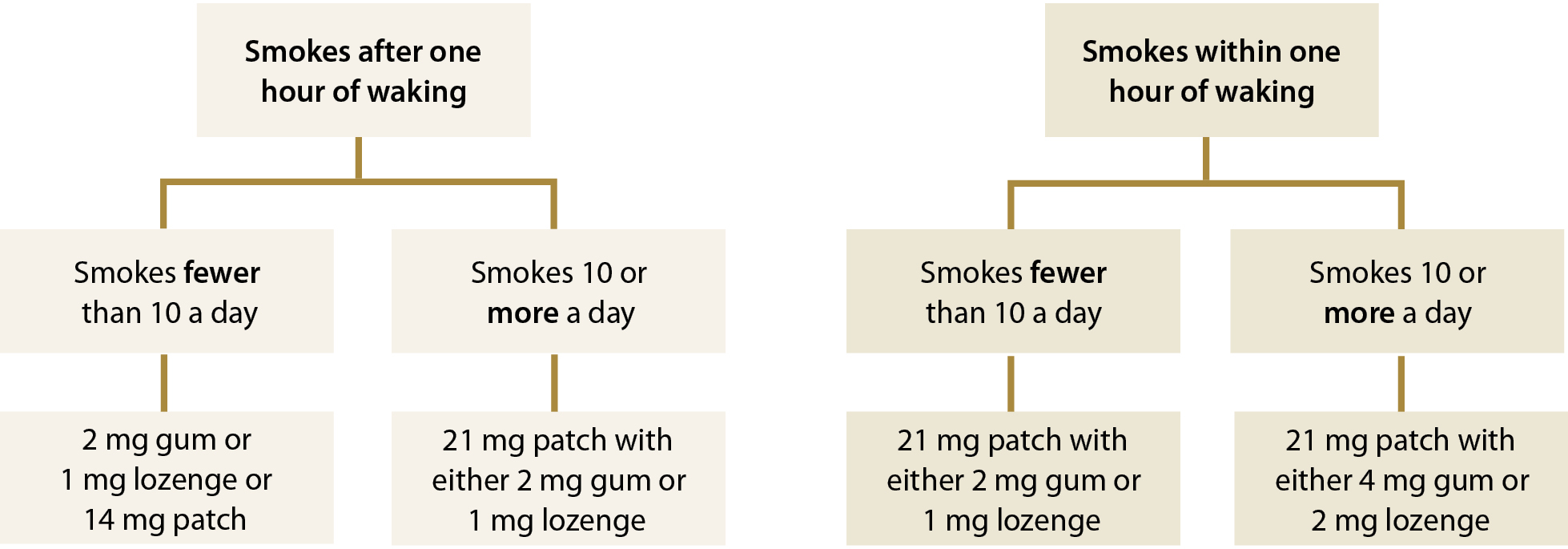
Figure 1: Nicotine dependence assessment algorithm for determining an appropriate NRT treatment regimen, adapted from Ministry of Health, 201421
Other smoking cessation medicines
Bupropion and nortriptyline are medicines used to aid smoking cessation which may be appropriate for people who have
previously tried to quit smoking with NRT or who prefer to quit using a medicine that does not contain nicotine.
Bupropion has a similar efficacy to NRT
Bupropion is an atypical antidepressant that reduces the desire to smoke by increasing the levels of dopamine and noradrenaline
in the brain as well as being a nicotinic acetylcholine receptor antagonist.20 It is thought that bupropion
blocks the effects of nicotine and elevates mood in people who are experiencing nicotine withdrawal.20
A Cochrane review found that NRT monotherapy and bupropion were equally effective as smoking cessation interventions.20 No
head-to-head studies comparing combination NRT with bupropion were identified,20 although it is likely that
combination NRT is more effective as a smoking cessation aid than bupropion as it has a similar efficacy to varenicline.
A review of 27 trials found insufficient evidence that adding bupropion to NRT provided any long-term benefit over treatment
with bupropion alone.25 If bupropion is taken in combination with NRT weekly monitoring of blood pressure
is recommended.26
Treatment with bupropion begins one to two weeks before the patient’s quit date.23 Initially, 150 mg bupropion,
daily, for three days, then 150 mg bupropion, twice daily (a maximum single dose of 150 mg, a maximum daily dose of 300
mg and a minimum of eight hours between doses), usually for seven weeks.23 The maximum daily dose for bupropion
for patients with risk factors for seizures and patients who are elderly is 150 mg.23
Bupropion is contraindicated in patients during acute alcohol or benzodiazepine withdrawal, or in patients with severe
hepatic cirrhosis, central nervous system tumour, a history of seizures, eating disorders, bipolar disorder or in patients
who have used monoamine oxidase inhibitors within the last 14 days.23 Patients using bupropion with any of
the following medicines may be at increased risk of seizures: antipsychotics, antidepressants, antimalarials, tramadol,
theophylline, systemic corticosteroids, quinolones and sedating antihistamines.23
The most common adverse effects associated with bupropion are insomnia in 30 – 40% of patients, dry mouth in 10% of
patients and nausea.25 Approximately one in 1 000 patients taking bupropion will experience a seizure.20
Nortriptyline is as effective as bupropion
Nortriptyline is a tricyclic antidepressant that increases the levels of noradrenaline in the brain. It has a more complicated
dosing regimen than bupropion.
A Cochrane review of six trials found nortriptyline was twice as effective as placebo as a smoking cessation medicine
and equally as effective as bupropion.25 There are no head-to-head comparisons of nortriptyline with NRT
or varenicline.25 There was insufficient evidence that adding nortriptyline to NRT provided any long-term
benefit.25
Nortriptyline is started ten to 28 days before a person attempts to quit smoking.23 Initially, nortriptyline
25 mg, daily, increased gradually over ten days to five weeks to 75 – 100 mg, daily, for up to six months.23 The
dose should be slowly tapered when the medicine is stopped.23
Common adverse effects of nortriptyline include dry mouth, drowsiness, light-headedness and constipation.25
Varenicline is subsidised as a second-line smoking cessation medicine
Varenicline is a partial nicotinic agonist which stimulates and blocks nicotinic acetylcholine receptors in the brain.3 This
reduces nicotine reward and causes a moderate and sustained release of dopamine in the brain,20 but not the
substantial increases associated with smoking tobacco.
Special Authority approval is required for subsidised treatment
Varenicline is fully subsidised with Special Authority approval for a maximum of three months use as a smoking cessation
treatment. To qualify for subsidised treatment a patient must:
- Be enrolled in, or about to enrol in, a smoking cessation programme which includes prescriber or nurse monitoring
- Have previously tried to quit smoking at least twice with NRT, with at least one of these attempts involving comprehensive
advice on the use of NRT, or the patient must have tried previously to quit smoking with bupropion or nortriptyline
- Not have used varenicline in the previous 12 months
- Have agreed that varenicline is not to be used in combination with other pharmacological smoking cessation treatments,
including NRT
How effective is varenicline as a smoking cessation medicine?
Varenicline is the most effective single formulation smoking cessation medicine subsidised in New Zealand.2 A
Cochrane review found that varenicline is more effective as a smoking cessation aid than any single NRT product or bupropion.20 However,
varenicline has a similar efficacy to combination NRT treatment.20
Initiating varenicline treatment
Varenicline treatment usually begins one to two weeks before the patient’s quit date with a two week “starter-pack”
followed by a ten week maintenance regimen:23
- Initially, varenicline 500 micrograms, once daily, for three days
- Increase to, varenicline 500 micrograms, twice daily, for four days
- Then, varenicline 1 mg, twice daily, for seven days until the starter pack is finished. During this period the patient
should stop smoking.
- Maintenance treatment of varenicline 1 mg, twice daily, for ten weeks
In patients with an estimated glomerular filtration rate (eGFR) < 30 mL/minute/1.73m2 the maintenance
dose of varenicline is 1 mg, once daily.23 Varenicline should be avoided by women who are pregnant or breast-feeding.23
When initiating treatment for varenicline consider offering the patient a quick follow-up consultation or phone call
from a practice nurse around the time of their quit date. This enables confirmation that the patient has successfully
completed the “starter pack” and to remind them to begin the maintenance treatment.
An additional 12 weeks of varenicline treatment increases the rates of continuous biochemically validated abstinence
from tobacco at 24 weeks by 1.42 times and at 52 weeks by 1.19 times.27 Treatment beyond 12 weeks with varenicline
is not subsidised in New Zealand.
Prescribe the “starter pack” and the maintenance treatment together
To ensure that patients receive the entire 12 week course of subsidised varenicline the prescription items for both
the starter and maintenance treatment can be included on the same form. Pharmacists will supply patients with the “starter
pack” and up to four weeks of initial maintenance treatment if a prescription for both items is presented. Patients will
need to return to the pharmacy to collect the remainder of their maintenance treatment. In 2014, almost 32 000 people
in New Zealand were dispensed varenicline from a community pharmacy, of whom over 2 200 were not dispensed the “starter
pack” and maintenance treatment on the same day, i.e. they may not have received a full course of treatment.28 If
a patient is prescribed a “starter pack” without maintenance treatment they should be contacted before this is finished
and offered another prescription for maintenance treatment.
Prepare patients for the adverse effects of varenicline treatment
To help patients taking varenicline complete the course of treatment they should be prepared for the possibility of
adverse effects before they begin. The “starter pack” allows the dose of varenicline to be increased slowly to reduce
the likelihood of adverse effects. In general, advise patients to contact the practice if they are considering stopping
treatment as many of the symptoms associated with treatment are manageable.
Nausea is the most common adverse effect of varenicline treatment
Nausea can be expected in 17% of patients taking varenicline and is generally mild, although in up to 8% of patients
it may be severe enough to cause discontinuation of treatment.20 If a patient taking varenicline reports
nausea emphasise the health benefits of quitting smoking and encourage them to continue with treatment. Patients may experience
less nausea if they take varenicline with food and a glass of water. Foods containing easily digested carbohydrates, e.g.
bananas or white rice, may be effective at reducing varenicline-induced nausea. An antiemetic, e.g. prochlorperazine or
metoclopramide, may be appropriate for patients experiencing moderate to severe varenicline-induced nausea, assuming there
are no interactions with any other medicines. The nausea associated with varenicline appears to be dose-dependent and
reducing the dose decreases its severity, although this also reduces the effectiveness of varenicline.20 A
reduced dose, e.g. varenicline 500 micrograms, twice daily, results in quit rates comparable with that achieved with NRT
alone or bupropion, and is preferable to the patient stopping treatment.27
Patients may experience sleep disturbances or headaches
Insomnia, abnormal dreams and headaches are associated with varenicline treatment.27 Patients who experience
mild to moderate adverse effects can be encouraged to finish the course of treatment as these relatively short-term effects
are likely to be outweighed by the long-term benefits of smoking cessation. Unlike the other adverse effects associated
with varenicline, headache does not appear to be dose-dependent and reducing the dose may not reduce this symptom.
Serious adverse psychological effects are rare
Varenicline treatment has been linked with serious psychological adverse effects. The evidence supporting an association,
however, is relatively weak and studies are confounded by the increased rate of suicide in people who smoke compared to
the general population.27 Patients taking varenicline should stop treatment and contact a health professional
if they notice negative changes in behaviour or thinking, mood swings, anxiety, depression or suicidal ideation.
In 2015, the United States Federal Drug Administration (FDA) found no association linking the use of varenicline with
neuropsychiatric disturbances, although it noted the low quality of the evidence prevented reliable conclusions from being
made.29 A recent study of over 51 000 patients in England who had taken varenicline found that it was actually
associated with a significantly reduced risk of depression and self-harm compared to treatment with NRT.30 From
April, 2007 to March, 2008, 3 415 patients in New Zealand were dispensed prescriptions for varenicline, 1 394 of whom
were surveyed about neuropsychiatric disturbances.31 Sleep disorders were reported by 4% of patients and
3% of patients reported depression (24 new-onset cases and 14 cases of worsening of existing depression).31 One
case of suicide, two cases of suicidal ideation and three cases of psychotic reaction were reported;31 it
is not known what the prevalence of these conditions are in people quitting smoking in New Zealand with other smoking
cessation medicines or among people who smoke in general.
Varenicline may reduce tolerance to alcohol
The FDA recently advised that varenicline may change the way people react to alcohol, including decreased tolerance
to alcohol, unusual or aggressive behaviour when drinking and memory loss.29 It is recommended that until
patients know how varenicline affects the way they react they should reduce their consumption of alcohol.29 Reducing
alcohol consumption will reduce the likelihood of smoking lapses in some people trying to quit.
Varenicline does not increase cardiovascular risk compared to NRT
There is limited evidence that the use of varenicline may be associated with a small increase in cardiovascular risk.
However, the authors of the recent study from England concluded that the use of varenicline was not associated with an
increased cardiovascular risk; compared to NRT it was associated with a significantly reduced risk of ischaemic heart
disease, cerebral infarction and heart failure.30
Preventing relapses in patients taking varenicline
The first two weeks of a quit attempt is a crucial time for patients taking varenicline. Patients who remain completely
smokefree during this time are significantly more likely to be adherent to treatment and achieve long-term abstinence
from smoking.32 A study of almost 700 people taking varenicline found that patients who were abstinent from
tobacco two weeks after quitting were 2.7 times more likely to be adherent to varenicline treatment than those who were
not completely smokefree at this point.33 This two week window is the best time to deliver behavioural support.
Varenicline in combination with NRT – unsubsidised but effective
A clinical trial that randomised 446 people trying to quit smoking with varenicline to either a nicotine patch or a
placebo patch found that varenicline in combination with NRT was more effective than varenicline with placebo.34 After
12 weeks of treatment, 55% of patients treated with varenicline and NRT were biochemically confirmed to be abstinent from
tobacco, compared to 41% of patients treated with varenicline and a placebo patch; statistically significant differences
persisted between these groups at 24 weeks and six months.34 There was no difference in adherence rates with
approximately 80% of patients in each group completing the treatment regimen.34
Patients who were treated with varenicline in combination with NRT were more likely to experience skin reactions, nausea,
sleep disturbances, constipation and depression, although only the increased rate of skin reactions was statistically
significant (14% versus 8%).34 The group treated with varenicline alone reported more abnormal dreams and
headaches.34
Varenicline is not subsidised if it is co-prescribed with NRT although some patients who want to maximise their chances
of quitting smoking may be prepared to pay for this combination treatment.
Patients taking antipsychotic medicines who quit smoking may need dose reductions
The cytochrome P450 enzyme CYP1A2 which metabolises some antipsychotics is induced by the hydrocarbons and tar-like
compounds in tobacco smoke.35 Patients who are taking certain antipsychotics, e.g. clozapine, olanzapine,
chlorpromazine and haloperidol, may have increased serum levels of these medicines following smoking cessation and therefore
will require dose reductions.35 There have been reports in the literature of serious adverse effects following
abrupt smoking cessation in people taking clozapine.36 Some smoking cessation medicines may not be appropriate
for patients with a history of mental disorders.
 For further information see: “
Smoking cessation beyond the ABC: Tailoring strategies to high-risk groups”, BPJ 64 (Oct, 2014).
For further information see: “
Smoking cessation beyond the ABC: Tailoring strategies to high-risk groups”, BPJ 64 (Oct, 2014).