View / Download pdf
version of this article
Paroxetine use by people under the age of 18 years
The Committee on Safety of Medicines of the UK Medicines and Healthcare products Regulatory Agency (MHRA) advises that “paroxetine
should not be used in children and adolescents under the age of 18 years to treat depressive illness” (Duff, 2003).
This advice was given because of lack of evidence of efficacy in this age group combined with an increased risk of suicidal
thoughts and self-harm compared with placebo.
The problem with increased suicidal ideation in this age group may extend across all SSRIs and indeed all antidepressants.
However fluoxetine is the only antidepressant that has evidence of benefit over placebo in children and adolescents and
therefore may have a positive benefit-risk profile. It is the only SSRI registered for use in people under the age of
18 years in USA and UK. None of the tricyclic antidepressants has ever been authorised for treatment of depressive illness
in children under the age of 16 years in UK or Europe. Non-pharmacological interventions, in particular CBT, are first
choice therapy in the management of depression in young people but may need augmentation by fluoxetine.
We recommend:
- Non-pharmacological measures are first line therapy for depression in young people.
- When an antidepressant is indicated in this age group fluoxetine is chosen.
- Paroxetine should not be prescribed as a new therapy for patients less than 18 years of age with a depressive illness.
- A 10-minute audit is performed to identify any patients less than 18 years of age taking paroxetine.
Reference: Duff G. Safety of seroxat (paroxetine) in children and adolescents under 18 years - contraindication in the
treatment of depressive illness 2003.
http://www.sehd.scot.nhs.uk/
Recommended further reading: Weller I. Report of the CSM expert working group on the Safety of selective Serotonin reuptake
Inhibitor antidepressants. Committee on Safety of Medicines, 2004. MHRA.
http://www.mhra.gov.uk/home/groups/pl-p/documents/drugsafetymessage/con019472.pdf
Using Medtech, patients on paroxetine under the age of 18 years can be identified easily by using the query builder.
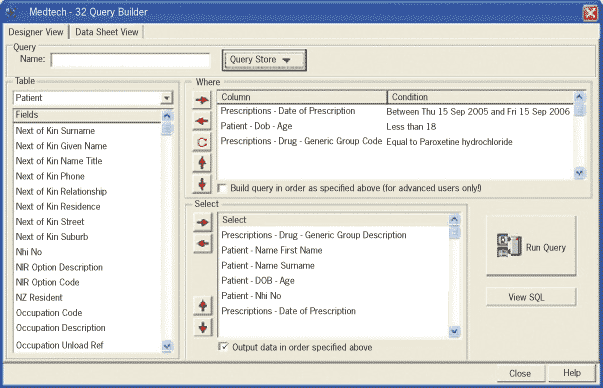
The query builder form should be completed as shown in the screen shot above by selecting items from the box on the
left of the screen to move to the appropriate box on the right. Change the date of the prescriptions to the 12 month period
prior to the current date.
When the boxes on the right look like the ones on our screen shot (except for the changed dates) click the button labelled ‘Run
Query’ to print your list of patients.
Once patients are identified for the audit:
- Carefully assess them for suicidal or hostile behaviour.
- If patients are not doing well on paroxetine, consider a change of treatment.
- Ensure you communicate with any other relevant healthcare providers about any changes.