Key practice points:
- Legislative changes now mean that medicinal cannabis products can be prescribed via two main pathways:
- Products that have been granted consent to distribute under the Medicines Act 1981 (i.e. “Medsafe approved”) can be prescribed by authorised prescribers*
- Products that are verified by the Medicinal Cannabis Agency as meeting the minimum quality standard but have not been granted Medsafe approval can be prescribed by a medical practitioner (i.e. a doctor). These prescriptions can either be supplied directly by the medical practitioner, or dispensed by a pharmacist under Section 29 of the Medicines Act.
- Community pharmacists should be familiar with the indications that medicinal cannabis might be appropriate for, what type of products are available and how they can be prescribed, so that they can have informed discussions with people enquiring about its use
- Upon receiving a prescription to dispense, pharmacists should ensure all appropriate legal and regulatory criteria are fulfilled before placing an order to procure a medicinal cannabis product; generic product substitutions are not permitted
- If a person has a valid prescription for a product that is Medsafe approved or verified as meeting the minimum quality standard, pharmacists can procure the named product from a wholesaler or manufacturer in accordance with any relevant controlled drug or unapproved medicine requirements (N.B. Cannabidiol [CBD] products are not controlled drugs)
- Medical practitioners can still prescribe medicinal cannabis products that are neither Medsafe approved nor verified as meeting the minimum quality standard, however, there are more restrictive access requirements:
- These products must be imported from overseas directly in an amount required for a named patient under the current care of the prescribing medical practitioner, or by a pharmacist on the medical practitioner’s behalf (with a valid prescription)
- If the product selected is a controlled drug (i.e. a non-CBD product), Ministerial approval to prescribe is also required, as well as a licence to import controlled drugs issued by Medsafe for each consignment
- Pharmacies should not advertise medicinal cannabis products to the public in any way, including product display and pricing information
- Medicinal cannabis can potentially be prescribed in forms intended for vaporising (but not smoking); there are no Medsafe approved cannabis vaporisers. However, vaporisers that have been approved as medical devices by an overseas regulator can be imported and sold legally in New Zealand.
*Section 2(1) of the Medicines Act 1981 defines an authorised prescriber as a nurse practitioner, an optometrist, a medical practitioner, dentist, a registered midwife or a designated prescriber. As of April, 2022, Sativex is the only medicinal cannabis product approved under the Medicines Act 1981. Ministerial approval is required to prescribe approved medicinal cannabis products that are controlled drugs. All registered medical practitioners (i.e. doctors) have been granted Ministerial approval to prescribe Sativex without the need to submit an application to the Ministry of Health (see: https://gazette.govt.nz/notice/id/2020-go997).
On 1 April, 2020, the Misuse of Drugs (Medicinal Cannabis) Regulations 2019 came into force, allowing the Medicinal Cannabis Scheme (The Scheme) to be implemented.1 The Scheme is administered by the Medicinal Cannabis Agency, which is part of the Ministry of Health, and aims to improve patient access to quality medicinal cannabis products in New Zealand by:1, 2
- Delivering a framework for a licensed domestic industry including the cultivation of cannabis crops, as well as the manufacture and supply of medicinal cannabis products. These products must be unadulterated and only contain active ingredients extracted from cannabis plants (not other plant varieties or synthetic cannabinoids).
- Setting a minimum quality standard that all medicinal cannabis products must meet. All medicinal cannabis products should be verified against this standard by the Medicinal Cannabis Agency; if verified, they can be supplied to a patient on prescription, without requiring Ministerial approval. However, the standard is not equivalent to Medsafe approval as there is no requirement for safety or efficacy data in the verification process. Instead, it recognises that a product is manufactured under strict good manufacturing practice requirements, and ensures product quality, consistency and reduces the risk of harmful contaminants being present. This assurance cannot typically be obtained for other unapproved medicines.
Before this shift in legislation, case-by-case approval was required from the Ministry of Health for a medicinal cannabis product to be prescribed, in addition to specialist recommendation. With the new Scheme, medicinal cannabis products can now be prescribed by any registered medical practitioner (i.e. doctor) to any patient for any indication within the scope of their practice (see: “How these changes affect doctors’ prescribing”).1
How these changes affect doctors’ prescribing
The new regulations mean that medicinal cannabis products can be prescribed to any patient via two main pathways (Figure 1):1, 4
1. Medicinal cannabis products granted consent to distribute under the Medicines Act 1981, i.e. “Medsafe approved” (or those with provisional approval), can be prescribed by an authorised prescriber* within the scope of their practice. Approved medicinal cannabis products, that are controlled drugs (e.g. Sativex), require Ministerial approval to prescribe.
*Section 2(1) of the Medicines Act 1981 defines an authorised prescriber as a nurse practitioner, an optometrist, a medical practitioner, dentist, a registered midwife or a designated prescriber. As of April, 2022, Sativex is the only medicinal cannabis product approved under the Medicines Act 1981. All registered medical practitioners (i.e. doctors) have been granted Ministerial approval to prescribe Sativex without the need to submit an application to the Ministry of Health (see: https://gazette.govt.nz/notice/id/2020-go997).
2. Unapproved medicinal cannabis products verified as meeting the minimum quality standard by the Medicinal Cannabis Agency can be prescribed by a medical practitioner (i.e. a doctor). These prescriptions can either be supplied directly by the medical practitioner, or dispensed by a pharmacist under Section 29 of the Medicines Act.
Click here for an up to date list of available medicinal cannabis products from the Ministry of Health website.
Other medicinal cannabis products can still be prescribed
It is still possible for doctors to prescribe unapproved medicinal cannabis products NOT verified as meeting the minimum quality standard by the Medicinal Cannabis Agency for supply under Section 29 of the Medicines Act (Figure 1). However, unless the product fits the definition of being a CBD product (see: “The criteria for being a ‘CBD product’”) it is a controlled drug. Therefore, approval for a named patient must first be obtained from the Minister of Health (delegated to the Ministry of Health) following an application from a relevant specialist or the Chief Medical Officer of a District Health Board. If this is granted, the controlled drug can only be used by the patient for the specified indication. In addition, there are more restrictive access criteria for these products (see: Figure 1 and Table 1).
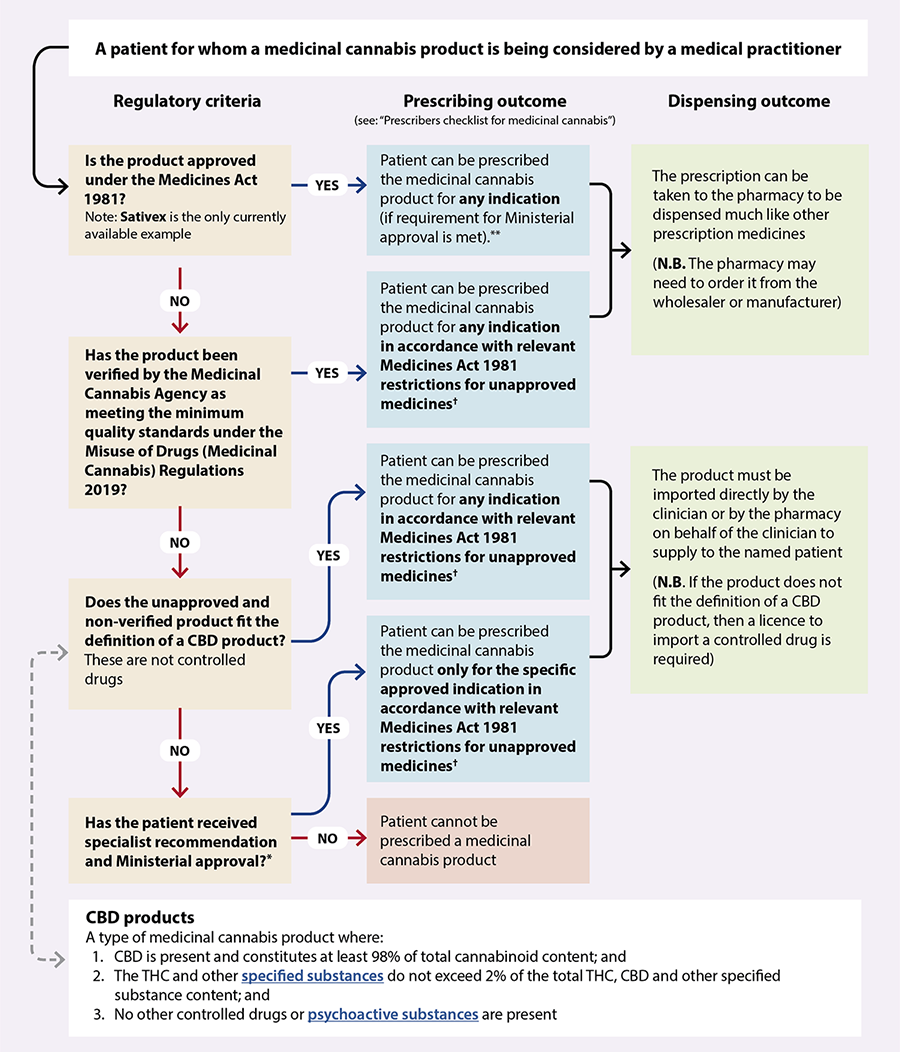
Figure 1. How the new regulatory framework affects the prescribing of medicinal cannabis products by registered medical practitioners (i.e. doctors) in New Zealand.* 1
*An
application
form for Ministerial approval to prescribe non-pharmaceutical grade medicinal cannabis without consent for
distribution is available on the Ministry of Health website. The application must be completed by a specialist
who is managing the condition that the product is intended to treat or by the Chief Medical Officer of a District Health Board.
† Unapproved medicines must be prescribed by a medical practitioner, and directly supplied to the patient by the prescribing medical practitioner, or dispensed by a pharmacy via Section 29 of the Medicines Act
**All registered medical practitioners (i.e. doctors) have been granted Ministerial approval to prescribe Sativex without the need to submit an application to the Ministry of Health
‡As of May 2022, Sativex is the only medicinal cannabis product approved under the Medicines Act 1981. All registered medical practitioners (i.e. doctors) have been granted Ministerial approval to prescribe Sativex without the need to submit an application to the Ministry of Health
(see: https://gazette.govt.nz/notice/id/2020-go997).
In addition, doctors are currently the only group that can prescribe unapproved medicines for supply under Section 29. Therefore,
it is expected that doctors will be the predominant group writing prescriptions for medicinal cannabis products.
While medicinal cannabis products require a medical practitioner’s prescription, community pharmacists may be approached by people who are interested in its possible benefits for treating their particular condition or symptom(s) and have questions about how to access it. These discussions may be a valuable starting point for some patients and save them an unnecessary trip to their general practice, or prompt them to make an appointment if treatment could potentially be suitable.
Medicinal cannabis is not a first-line choice for any indication
There are a number of potential indications for medicinal cannabis, including:9–13
- Chronic neuropathic or malignant pain*, or pain from other causes in a palliative care-setting
- Chemotherapy-related nausea and vomiting
- Refractory spasticity associated with multiple sclerosis
- Seizures due to epilepsy
However, given the absence of large and robust randomised controlled trials (RCTs) comparing medicinal cannabis with established treatments, these products are not considered to be first-line for any indication. In general, trialling a medicinal cannabis product is only suitable if (1) someone experiences ongoing symptoms despite optimal dosing of available evidence-based treatments, or (2) conventional treatments are contraindicated or not tolerated.
*A 2023 Cochrane Systematic Review shows that medicinal cannabis provides no clinically relevant benefit for patients with malignant pain compared with placebo. See update box in the main article.
For further information on the indications for medicinal cannabis use, see: “An overview of medicinal cannabis for health practitioners”
How THC and CBD differ
Cannabinoids are a class of chemically related compounds capable of interacting with human cannabinoid receptors. This interaction can elicit a wide range of biological effects throughout different body systems.5, 6 The most notable phytocannabinoids (i.e. plant-derived cannabinoids) are tetrahydrocannabinol (THC) and cannabidiol (CBD):5, 6
- THC – the primary psychoactive cannabinoid in Cannabis sativa; alongside its derivatives, THC is responsible for the “high” experienced with cannabis use due to CB1 receptor binding in the brain and central nervous system. In addition, binding of THC to endocannabinoid receptors throughout the body can cause numerous other effects, e.g. elevated heart rate, dizziness, impaired reaction time, reduced concentration and other neurological and behavioural effects.
- CBD – a non-intoxicating cannabinoid, which is thought to modulate some of the psychoactive effects associated with THC consumption. Although it is considerably less likely to produce a “high” or intoxication in isolation, it still can exert neuromodulatory functions.
The criteria for being a “CBD product”
A CBD product is a subtype of medicinal cannabis product that has a cannabinoid content of at least 98% CBD. The remaining cannabinoid content (up to 2%) may include THC or other specified substances,* but must not contain any non-cannabinoid psychoactive substances or other controlled drugs in the formulation.7
For example:
- A medicinal cannabis product with 39 mg total CBD, and 0.7 mg total THC and other related psychoactive substances, would have a non-CBD cannabinoid content of 1.76% – this meets the definition of a CBD product
- A medicinal cannabis product with 38 mg total CBD, and 1.7 mg total THC and other related psychoactive substances, would have a non-CBD cannabinoid content of 4.28% – this does not meet the definition of a CBD product
Under the Misuse of Drugs Amendment Act 2018, CBD and CBD products are no longer classified as being controlled drugs.7 However, they are designated as prescription medicines under Schedule One of the Medicines Regulations 1984. Unless a CBD product is consented under the Medicines Act 1981, it is an unapproved medicine and can only be prescribed by a medical practitioner (i.e. doctor) and directly supplied to the patient by the prescribing medical practitioner or dispensed by a pharmacist under Section 29 of the Medicines Act.8
*“Specified substances” refers to a list of compounds that naturally occur in cannabis, capable of inducing more than a minor psychoactive effect, by any means, in a person; THC is just one example. For more information on what constitutes a specified substance, see Section 2A of the Misuse of Drugs Act 1975. If you require clarification as to whether a particular ingredient qualifies as a specified substance, email the Medicinal Cannabis Agency: [email protected]
Framing an initial discussion around medicinal cannabis products
If a person has questions regarding medicinal cannabis, a general approach for community pharmacists to guide the discussion could be:
- Establishing that the person understands the difference between illicit and medicinal cannabis
- Checking they are aware that the minimum quality standard is not equivalent to a product being Medsafe approved, i.e. there is no requirement for safety or efficacy data in the verification process
- Identifying what the person’s symptoms are, and what medicines, if any, the person has used previously to alleviate them
- Consideration of over-the-counter medicines, non-pharmacological treatments or potential prescription medicines that may be suitable for the patient and have more evidence of benefit
- Discussing whether they have a relevant indication for medicinal cannabis use; ultimately, the patient’s doctor will make this decision (and it is important not to raise patient expectations that a prescription will be made), however, there are certain indications that are very unlikely to be suitable, e.g. short-term pain
- Considering any circumstances the person reports that are likely to make medicinal cannabis use unsuitable, e.g. pregnancy, a history of psychiatric disorders, unstable cardiovascular or cardiopulmonary disease if information is available
- Referring the patient to a general practitioner if medicinal cannabis might be appropriate as a treatment option, or if the patient has further questions about medicinal cannabis
Step 1: check that it meets the requirements
When presented with a prescription for a medicinal cannabis product, pharmacists should ensure all appropriate legal and regulatory criteria are fulfilled before placing an order to procure it.
In New Zealand, all medicinal cannabis prescriptions must:1
- Specify the brand and prohibit any generic substitutions
In some cases, prescribing software used in general practices can automatically enable generic substitutions by default; if this is the case, ensure a generic substitute is not dispensed and inform the prescribing clinician. Likewise, if routine processes exist within the pharmacy allowing generic substitutions, ensure that all staff understand that these do not apply to medicinal cannabis products.
- Not be for a product in a form intended for smoking (vaporising is permitted)
- Not be for a product meeting the definition of “food” under the Food Act 2014
- Not be for a product in a sterile dosage form, e.g. eye drops
- Be for no more than a three-month supply if it is a CBD product
Additional requirements for medicinal cannabis products that are controlled drugs, e.g. Sativex (N.B. CBD products are not classified as controlled drugs):
- Be handwritten on a controlled drug prescription form, or on a personally signed and barcoded controlled drug ePrescription
For controlled drug prescriptions, pharmacists should verify the prescription if the signature of the prescriber is unknown to them.14
- Be for no more than a one-month supply
- Be accompanied by a Ministry of Health approval number,* in accordance with Regulation 22 of the Misuse of Drugs Regulations 1977, if the product:
- Is a controlled drug; and
- Not Medsafe approved; and
- Not verified as meeting the minimum quality standard
* This approval number will be detailed on the approval letter sent to the prescriber from the Ministry of Health. Pharmacist should check to make sure that each prescription has a valid written approval number prior to procuring and dispensing the prescribed product.
Further considerations. Although contraindications should already have been considered by the prescribing doctor, it is important to check that the patient does not have a history of hypersensitivity to ingredients in the specific product. Cannabinoids in medicinal cannabis products are often suspended in carrier oils, e.g. Sativex contains peppermint oil. This is also a good opportunity to confirm that the person is not taking any medicines (prescribed or over-the-counter) that have the potential to interact with cannabinoids; in some cases, people may be taking interacting medicines prescribed by another clinician (i.e. other than the doctor who wrote the prescription for medicinal cannabis).15
The New Zealand Formulary (NZF) interaction checker can be used to assess whether a patient’s current medicines have potential interactions with CBD alone, CBD + THC or marijuana (Cannabis sativa). For further information, see: https://nzf.org.nz.
After ensuring the medicinal cannabis prescription is valid, the next step for community pharmacists will be procuring the specified product. This process depends on a number of factors, including its Medsafe approval or minimum quality standard verification status, and whether it is manufactured in New Zealand (Table 1).
Table 1. Information for pharmacy procurement of medicinal cannabis products.1
| Product type |
How to procure the product |
Notes |
| Medsafe approved |
- Medsafe approved products can be ordered from pharmaceutical wholesalers directly (information on suppliers is listed on the Ministry of Health website)
- As of April, 2022, the only medicinal cannabis product that has Medsafe approval is Sativex
|
- These products can be procured and stocked by pharmacies in the absence of a prescription, i.e. to build up supply in anticipation for repeat prescriptions; however, advertising them to the public is not permitted (see: “Products and prices cannot be displayed in the pharmacy”)
|
| Not Medsafe approved, but has been verified as meeting the minimum quality standard |
- Products that are verified and manufactured in New Zealand can be procured directly from the domestic manufacturer
- Products that have been verified but manufactured overseas can be imported by New Zealand wholesalers; to procure these products, contact the licence holder listed on the Ministry of Health website
|
- These products can be procured and stocked by pharmacies in the absence of a prescription, i.e. to build up supply in anticipation for repeat prescriptions; however, advertising them to the public is not permitted (see: “Products and prices cannot be displayed in the pharmacy”). The supply of these products needs to comply with Section 29 requirements.
|
| Not Medsafe approved and has not been verified as meeting the minimum quality standard |
- These products must be imported into New Zealand directly in a quantity required for a named patient from the overseas manufacturer. Having an expectation of future prescriptions is not considered to be a reasonable excuse to import medicinal cannabis products that have not been verified against the minimum quality standard.
- Import can be undertaken by the prescribing doctor themselves once they select a specific branded product, but in some instances they may request that a pharmacist imports the named product on their behalf
|
- A prescription is required for each import which must be supplied to Border Control; if ongoing use is anticipated, the prescribing doctor and pharmacy will need to discuss a plan for the timing of prescriptions and import requests to ensure the continuity of supply
- CBD products do not require any additional licences for import; however, a certificate of analysis should be requested from the manufacturer to confirm that the product meets the New Zealand definition of being a CBD product. This should accompany the imported product to assist New Zealand border officials in their assessment.
- For other medicinal cannabis products (excluding CBD products), Ministry of Health approval should already have been obtained (as they are controlled drugs), and a licence to import controlled drugs is required for each consignment, issued by Medsafe (for further information, email Medicines control); the pharmacy will need to have already received a prescription from the prescriber with Ministerial approval before applying for this import licence
|
The advertising of medicinal cannabis products
Products and prices cannot be displayed in the pharmacy
The advertising of unapproved medicinal cannabis products and controlled drugs to the public is not permitted; this includes information about their price, availability or visible display of the product. These restrictions apply to anyone, including manufacturers, suppliers, wholesalers, pharmacies and healthcare practitioners. Controlled drugs that are approved medicines (e.g. Sativex) can be advertised to doctors or pharmacists if it is in a publication that is distributed solely or mainly to practitioners or pharmacists.14 For pricing information relating to unapproved medicinal cannabis products, the manufacturer or wholesaler should be contacted directly. Further information is available from the Ministry of Health website.
Product costs will likely decrease over time, but inequitable access is likely to continue
Medicinal cannabis products are not funded by PHARMAC, and many people will consider the current pricing to be unaffordable. However, given that the Medicinal Cannabis Scheme permits increased competition associated with domestic cultivation, manufacturing and supply, the average cost of medicinal cannabis products is expected to decrease over time. For the majority of products, the pricing of medicinal cannabis will be set by the pharmacies, and may reflect a number of factors, e.g. domestic manufacturer pricing and competition, the pricing arrangement between the pharmacy and the supplier, and public demand for products.
Anecdotal reports have indicated that the cost of some unapproved medicinal cannabis products (particularly CBD products) has reduced following the shift in legislation. However, the reduction in pricing will not necessarily make ongoing medicinal cannabis use any more realistic to those experiencing financial hardship. Given the lack of funding and substantial costs associated with ongoing prescriptions, inequitable access is likely to continue. Therefore, if a person is interested in medicinal cannabis, having a general discussion about the associated costs is important as it allows them to consider its affordability based on their specific financial circumstances and priorities.
Medicinal cannabis devices
Under the new legislation, both dry cannabis flower and oil extracts are permitted as dosage forms and these can potentially be consumed using a medical vaporiser.1, 2 Smoking cannabis continues to not be permitted.1, 2 Although there are currently no Medsafe approved cannabis vaporisers available, the Misuse of Drugs (Prohibition of Utensils) Notice 2020 enables the import and sale of vaporisers that have been approved as medical devices by an overseas regulator.16 Other cannabis vaporiser devices, and unregulated utensils with prohibited features (e.g. a bong, hash pipe or a roach clip with a pincer or tweezer action), continue to be prohibited from New Zealand and may be confiscated by Customs.16 The importer needs to provide evidence that the device is an approved medical device in another jurisdiction, otherwise Customs may need to contact the Medicinal Cannabis Agency for confirmation.
In order for any imported device to be legally supplied, it needs to be registered in the Web Assisted Notification of Devices (WAND) database (this condition does not apply for personal imports). Devices can be advertised in the pharmacy as being for the use of medicinal cannabis in general, however, care should be taken to not implicitly advertise the availability of unapproved medicinal cannabis products.
If a medicinal cannabis device is advertised in a pharmacy and manufactured under the same brand as an unapproved medicinal cannabis product, then this could potentially be a breach of the advertising restrictions.
Labelling
In addition to meeting the packaging and labelling requirements for medicines outlined in the Medicines Regulations 1984, pharmacists should be aware that the manufacturers of medicinal cannabis products are required to:2
- Clearly detail the CBD and THC content (including their corresponding acid derivatives), as well as any other ingredient derived from cannabis whose content is at least 1% of the total weight or volume if they have been verified against the minimum quality standard
- Include the statement “MEDICINAL CANNABIS PRODUCT” on the principal display panel
N.B. These requirements do not need to be included on the pharmacy label when medicinal cannabis products are re-packed for dispensing.
Storage
Medicinal cannabis products may have specific storage requirements; these will be detailed either on the medicine datasheet or in the manufacturer’s instructions. For Sativex, the datasheet specifies that it must be stored between 2 – 8°C, i.e. refrigerated.14 When a medicinal cannabis product is dispensed to a patient, they should be informed of these requirements, and advised to keep it in a secure location that children cannot easily access, e.g. a high shelf or locked cupboard.
Updated instructions may be issued over time. For products that have recently been verified as meeting the minimum quality standard, manufacturers may only have had data to support short shelf-life stability with their initial application. If data on long-term stability becomes available, storage recommendations may change.17 Therefore, it is important to re-check manufacturer’s instructions each time a product is received, rather than relying on information from a previous procurement.
Controlled drug storage. In general, medicinal cannabis products that are controlled drugs (i.e. non-CBD products) must be stored within the pharmacy in a locked safe/compartment that meets the requirements outlined in Regulation 28 of the Misuse of Drugs Regulations 1977, e.g. constructed of metal or concrete (or both), secured to or form part of the building. However, amendments to these Regulations make an exception for pharmacists to avoid such security requirements if the medicinal cannabis product:14
- Contains CBD (any amount); and
- Contains ≤ 27 mg/mL THC; and
- Has consent for distribution under the Medicines Act 1981; and
- Requires refrigeration to ensure its efficacy
As of April, 2022, only Sativex meets this criteria, i.e. it can be stored in a fridge that does not need to be locked and secured to the building, despite being a controlled drug.
Once the medicinal cannabis product has been procured, it can be dispensed in accordance with the prescription. Pharmacists should confirm that the person understands any recommended medicine regimen (e.g. dose, frequency and duration) and advise them to consult with their doctor if their symptoms worsen or they have not met their treatment objectives in an agreed timeframe.
If it is a controlled drug. Restrictions around the dispensing of controlled drugs apply. For example, prescriptions relating to Class B controlled drugs (e.g. THC) cannot be dispensed more than seven days after the date of the prescription.14 Considering this brief timeframe, it is likely that a new prescription will need to be obtained from the prescribing medical practitioner in order for a pharmacist to dispense imported products that are controlled drugs (or the original prescription can be sent back to the medical practitioner to have the date changed and then returned to the pharmacy). Dispensed prescriptions must be recorded in a Controlled Drugs Register. Pharmacies must retain the pharmacy copy of dispensed controlled drug prescriptions for four years, and Controlled Drugs Registers for four years after the date of last entry.14
If it is an unapproved medicine. Ensure that any requirements for supply under Section 29 of the Medicines Act 1981, are fulfilled:8, 18
- If the pharmacy is the importer of the medicine, information on the prescribing doctor and patient, the medicine, the date and the place where the medicine was sold or supplied must be kept by the pharmacy
- Monthly notifications of supply volumes must be made to Medsafe using the form on the Medsafe website
- If the medicine is procured from a wholesaler, information about the supply must be provided to them for forwarding back to the importer. The importer is responsible for supplying the information requirements under Section 29 back to Medsafe.
For further information on medicinal cannabis – including indications, cautions, and other factors that affect prescribing decisions – see the main resource for health practitioners: “An overview of medicinal cannabis for health practitioners”.





