Dermatoscopy
Dot: Structure too small to describe its shape
Chaos: More than one pattern in a pigmented lesion associated with asymmetry of colour, structure or
border abruptness
Clod: Defined structure (i.e. not a line) that can be any shape or colour
Clues: Used as part of the Chaos and Clues dermatoscopic method of pattern analysis to determine whether
a lesion should be excised. In a lesion displaying chaos, if there are one or more clues to malignancy, the lesion should
be excised. Clues to malignancy in a pigmented lesion are: grey/blue structures, eccentric structureless areas, thick lines
that are reticular or branched, peripheral black dots/clods, segmental radial lines or pseudopods, white lines, polymorphous
vessels and large polygons. Parallel lines on ridges are a clue on palms and soles, and chaotic lines are a clue on nails.
Pattern: An arrangement of lines (most often reticular, but can be branched, parallel or radial), dots,
clods or structureless zones
Pseudopod: A radial line with a bulbous end
Reticular pattern: A complete or incomplete network of intersecting lines
Revised pattern analysis: A system of describing the patterns and features of a pigmented lesion according
to structures, colour and symmetry. A modification of the revised pattern analysis is called Chaos and Clues; revised pattern
analysis of a lesion shows chaos, the specific features of the lesion reveal clues to malignancy.
Structureless zone: Lacks lines, dots or clods
Common benign skin lesions
Angioma: Benign vascular skin lesion, usually < 1 cm in diameter. Angiomas often appear black to the
naked eye, however, dermatoscopic examination may show red, purple or blue clods.
Atypical naevus: Characterised by at least three of the following: size > 5 mm, ill-defined or blurred
border, irregular margin resulting in an unusual shape, colour variation - mostly pink, tan, brown or black, flat and bumpy
components. Atypical naevi often arise on the mid-upper back, breasts and genital areas. Dermatoscopy may show reticular,
globular, homogenous or regression structures, an irregular vascular pattern and grey-blue areas.
Dermatofibroma: Benign fibrous nodule, often solitary and on the limbs. Most often a firm papule or nodule < 1.5
cm in diameter and may be pink or brown, often with a hypopigmented centre. Dermatofibromas are usually asymptomatic; however,
they can also be painful, tender or itchy. The most common dermatoscopic feature is a whitish centre with fine radial lines
or diffuse pigment fading peripherally.
Lentigo: Pigmented, flat or slightly raised lesion with a clearly defined border. May have a smooth or
slightly dry surface. The solar lentigo is found on sun exposed sites. A solitary facial solar lentigo can at times be difficult
to distinguish from melanoma in situ. Dermatoscopy shows a sharp, often scalloped border and light brown pigment
with subtle structures.
Melanocytic naevus: Often referred to as a mole. Benign skin lesion of developmental origin; congenital
or arises in childhood or early adult life within the dermis, at the junction of dermis and epidermis or both. Naevi vary
widely in appearance and type. Dermatoscopy shows symmetry of patterns; on most body sites this can be structureless, reticular
in a junctional naevus, aggravated clods in a dermal naevus or a combination of reticular and clod patterns in a compound
naevus. Site-specific characteristics of junctional naevi include a pseudoreticular pattern in facial naevi and a parallel
furrow pattern in acral naevi, i.e. palms, soles, fingers or toes.
Seborrhoeic keratosis: Benign keratinocytic proliferation often brown, black or tan but can also be pale,
yellow or have several colours. Seborrhoeic keratoses vary widely in appearance, e.g. they can be solitary or multiple,
flat or raised papules or plaques with a smooth, waxy or warty surface and range in diameter. As they are often irregular
in structure and colour and change over time, they can be confused with melanoma. They commonly have a sharp border around
the lesion that results in a stuck-on appearance. On sun exposed sites, a seborrhoeic keratosis may evolve within a solar
lentigo. Dermatoscopy may show scattered irregular, superficial yellow, orange, grey, brown or blackish clods, scattered
round or oval white or light-yellow clods (both best seen with non-polarised light) and thick curved lines forming a brain-like
or seaweed pattern.
Pre-cancerous lesions and non-melanoma skin cancers
Actinic keratosis: Also known as solar keratosis. Actinic keratosis is a pre-cancerous keratinocytic
(scaly) flat or thickened papule or plaque located on habitually sun exposed sites. They may be red or pigmented and patients
may report tenderness or be asymptomatic. On facial sites, dermatoscopy shows prominent follicles in a red background, known
as the strawberry pattern. Pigmented actinic keratosis also shows superficial angulated brown lines.
Basal cell carcinoma: Locally invasive keratinocytic skin cancer that varies in size, appearance and
histology, but tends to have a rolled border. Often described as a slow growing, pink or pigmented shiny plaque or nodule
that spontaneously bleeds or ulcerates (Figure 2). May invade deeply but rarely metastasises. Dermatoscopy on pigmented
lesions may show blue ovoid structures, grey dots, leaf-like converging radial lines and polarised white structures on a
shiny background of stroma (pale, pink, light brown or grey). Dermatoscopy on non-pigmented basal cell carcinomas may show
polarised white structures and vessels are often irregular, linear or branched.
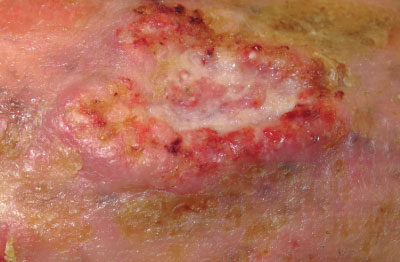
Cutaneous squamous cell carcinoma: Invasive keratinocytic skin cancer typically described as an enlarging
scaly or crusted ‘volcano-like’ nodule or irregular infiltrated plaque. Squamous cell carcinomas grow over weeks to months
and are often tender or painful and may ulcerate (Figure 3). Dermatoscopy generally shows a central
scale/crust and peripheral white structures or structureless zones. A pink colour and polymorphous vessels are most often
seen in fast-growing tumours.
Cutaneous squamous cell carcinoma in situ: Also known as intraepidermal squamous cell carcinoma.
A red or pigmented, slowly growing, scaly plaque; often multiple (Figure 4). Most common on sun exposed
sites but may arise anywhere. Dermatoscopy shows clusters of rounded vessels (red dots, clods, coils); vessels and pigment
are often in linear array.
Melanoma and excision terminology
Melanoma: The malignant proliferation of melanocytes usually within the epidermis (in situ)
or dermis (invasive). Most often arises from normal skin but approximately one-third arise within a melanocytic naevus (precursor
lesion). Dermatoscopic features are characterised by chaos and one or more clues to malignancy. Also see “Subtypes
of melanoma”.
Narrow complete excisional biopsy: An excision biopsy with 2 mm margins, that encompasses the entire
lesion and is of sufficient depth to avoid transection at the base.5
Wide local excision: Re-excision including an extra margin of tissue that is removed from the original excision
site following a positive pathology report for melanoma. Margins vary depending on Breslow thickness (distance of the deepest
point of the tumour from the granular layer of the epidermis).
For further information and for images of specific lesions, see: https://dermnetnz.org/
 |
 |
 |
Figure 2:
Nodular basal cell carcinoma (image supplied by DermNet NZ). |
Figure 3:
Poorly differentiated and ulcerated squamous cell carcinoma (image supplied by DermNet NZ). |
Figure 4:
Cutaneous squamous cell carcinoma in situ (image supplied by DermNet NZ). |





