Since 2010 the availability and use of the direct oral anticoagulants (DOACs) such as dabigatran has increased substantially in New Zealand.
The number of patients taking dabigatran has doubled in the past two years and 51% of patients taking oral anticoagulants are now treated with
dabigatran (46,170 patients).1 Dabigatran is renally excreted and therefore patients need regular monitoring due to an increased risk in bleeding
in those with reduced renal clearance.
- It is now recommended that all patients receive at least annual monitoring of renal function, using the Cockcroft-Gault
equation to calculate creatinine clearance
- More frequent monitoring is recommended in patients with reduced renal function, i.e. a creatinine clearance less
than 50 mL/min, or in situations where renal function may decline rapidly e.g. dehydrating illness, initiation of
a diuretic or hypovolaemia
- Declining renal function may necessitate a reduction in dose or even treatment withdrawal
Renal function should be monitored in patients taking dabigatran
Dabigatran is primarily renally excreted and is contraindicated in patients with a creatinine clearance
< 30 mL/min. It should be used with caution in patients with creatinine clearance between 30 and 50 mL/min,2 and in older patients.
It is therefore recommended that renal function is assessed:
- Prior to initiation of dabigatran (baseline testing)
- At least annually for ALL patients (ongoing monitoring)
- At least three to six monthly in those who have a creatinine clearance of 30 – 50 mL/min (ongoing monitoring), or
those who have had changes in their clinical condition/situation
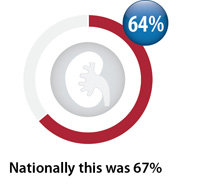
Nationally, over the last two years there has been a slight increase in baseline monitoring (from 64% to 67%),
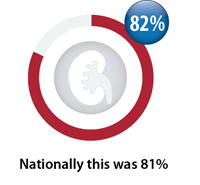
however ongoing monitoring has slightly decreased (from 82% to 81%).
|
Patients taking dabigatran
Total number of registered patients dispensed dabigatran between April, 2016 - March, 2017:
33
|
Patients initiated on dabigatran in this time period:
12
Patients with ongoing dabigatran dispensings in this time period:
21
|
|
Baseline creatinine testing
Percentage of registered patients initiated on dabigatran between April, 2016 – March, 2017 with a
creatinine test within three months prior and three weeks after first dispensing*
|
Ongoing monitoring of creatinine
Percentage of registered patients taking dabigatran on an ongoing basis with a creatinine test between April, 2016 – March, 2017
|
*For the purposes of this report, baseline testing was defined as a creatinine test in the three months prior to a first dispensing of
dabigatran, and also included tests performed in the three weeks after to account for patients who collected their prescription first. Clinicians may
have a small number of patients with stable renal function for whom a creatinine test more than three months prior is considered a satisfactory
baseline measure. Some patients may also receive baseline testing in a hospital setting, which is not included in this report.
- National Pharmaceutical Collection, 2017
- New Zealand Formulary, 2017. Availble from www.nzf.org.nz
Further reading: "The safe and effective use of dabigatran and warfarin in primary care" available from
www.bpac.org.nz/2017/anticoagulants.aspx
Further investigation of your prescribing
Undertaking an audit or peer group discussion may provide more detail to help identify
similarities and differences in prescribing practice compared
to other primary care practitioners. If any issues have been identified these resources can help instigate change, leading to more appropriate use of
medicines and facilitate best practice.
Feedback
We are always trying to improve our reports therefore we would like to know how useful you find them.
We would also like to hear if you have any further suggestions for presenting and comparing annual prescribing data.
Email us at: [email protected]





