Published: 25th November, 2024
Key practice points:
- Testosterone levels in males generally decline from middle age, however, the rate of decline is variable and influenced by many factors including obesity, co-morbidities, lifestyle and medicines
- The number of older males prescribed testosterone continues to rise in New Zealand; there is concern that some of this use may not be appropriate
- Investigation of testosterone levels (and consideration for treatment) is only indicated in patients with specific symptoms of hypogonadism that are impacting their quality of life, e.g. decreased libido and sexual function
- Hypogonadism is diagnosed based on at least two early morning serum total testosterone levels below the accepted threshold of normal in a patient with multiple features consistent with low testosterone
- Further testing may be required if diagnosis is uncertain; discuss with an endocrinologist
- If low testosterone is detected, first identify and address any modifiable causes
- Before prescribing testosterone discuss adverse effects, monitoring requirements, the uncertain long-term risks and need for life-long treatment
- Potential adverse effects of testosterone include venous thromboembolism due to secondary polycythaemia, worsening of lower urinary tract symptoms, acceleration of previously undetected or existing prostate cancer, impaired fertility and other androgenic effects
- Cardiovascular disease risk with testosterone replacement treatment is still uncertain. Recent short-term studies (i.e. fewer than three years follow up) have shown no increase in cardiovascular risk, however, long-term risk has not been well established; caution is still advised.
- A six-month trial of testosterone is recommended; if there is no benefit at six months, treatment should be stopped. N.B. Maximal therapeutic effect may not be obtained until 12 months of treatment.
- Most patients can be initiated on daily transdermal testosterone gel as it allows dose modification and abrupt withdrawal, if required
- Regular follow-up is required to evaluate clinical response and adverse effects. Recommended monitoring includes serum testosterone levels, prostate specific antigen and full blood count.
What’s changed?
This is a revision of a previously published article Prescribing testosterone in ageing males (BPJ 69, August 2015). Changes include:
- General article review and update of evidence
- Updated testosterone dispensing data for New Zealand between 2018 – 2023
- Review of evidence of cardiovascular risk in older males with hypogonadism prescribed testosterone replacement treatment; risk may be less than previously thought, but this is based on a treatment duration of three years or less and a cautious approach is still recommended
- Obstructive sleep apnoea is no longer a contraindication for initiating testosterone replacement treatment
- Inclusion of testosterone gel as a funded option in New Zealand and the discontinuation of funded testosterone patches
Testosterone is mainly prescribed for hypogonadism in New Zealand but is used in other clinical situations that are outside the scope of this article, e.g. gender affirming hormone treatment. The term “male” in this article refers to people whose biological sex is male and who are not taking oestrogen.
Testosterone is the primary circulating sex hormone in males, controlling sperm production, development and maintenance of typical secondary sex characteristics and influencing sexual function.1, 2 In adult males, testosterone is mainly produced in the testes, with a small proportion produced in the adrenal glands (Figure 1).1
Almost all testosterone in the body (total testosterone) is bound to sex hormone-binding globulin (SHBG) or weakly bound to albumin and other serum proteins; only 1 – 2% of total testosterone is non-bound and biologically active, i.e. free testosterone.1 The amount of SHBG in circulation influences free testosterone levels.3 See Table 1 for factors that affect SHBG.
Table 1. Conditions, medicines and clinical features that affect SHBG levels.4, 5
|
Increase SHBG
|
Decrease SHBG
|
|
Ageing
Anorexia
Anticonvulsant medicines
HIV
Hepatic disease, e.g. cirrhosis, hepatitis
Hyperthyroidism
Thyroid replacement hormone (levothyroxine)
Smoking
|
Acromegaly
Cushing’s disease
Glucocorticoids
Hypothyroidism
Nephrotic syndrome
Obesity
Testosterone supplementation
Type 2 diabetes
|
HIV = human immunodeficiency virus; SHBG = sex hormone-binding globulin
Defining hypogonadism
Hypogonadism is characterised by dysfunction of the testes, meaning the body cannot produce enough testosterone to carry out normal physiological processes.2 Hypogonadism can have an early or late onset, and can result from low or absent testicular testosterone production despite increased stimulation from the pituitary gland (primary or hypergonadotropic hypogonadism), inappropriate output from the hypothalamus and pituitary (secondary or hypogonadotropic hypogonadism) or a combination of the two.3, 4 The effects of hypogonadism can be reversed with exogenous testosterone (i.e. testosterone replacement treatment), however, the success of this approach depends on the specific clinical situation.
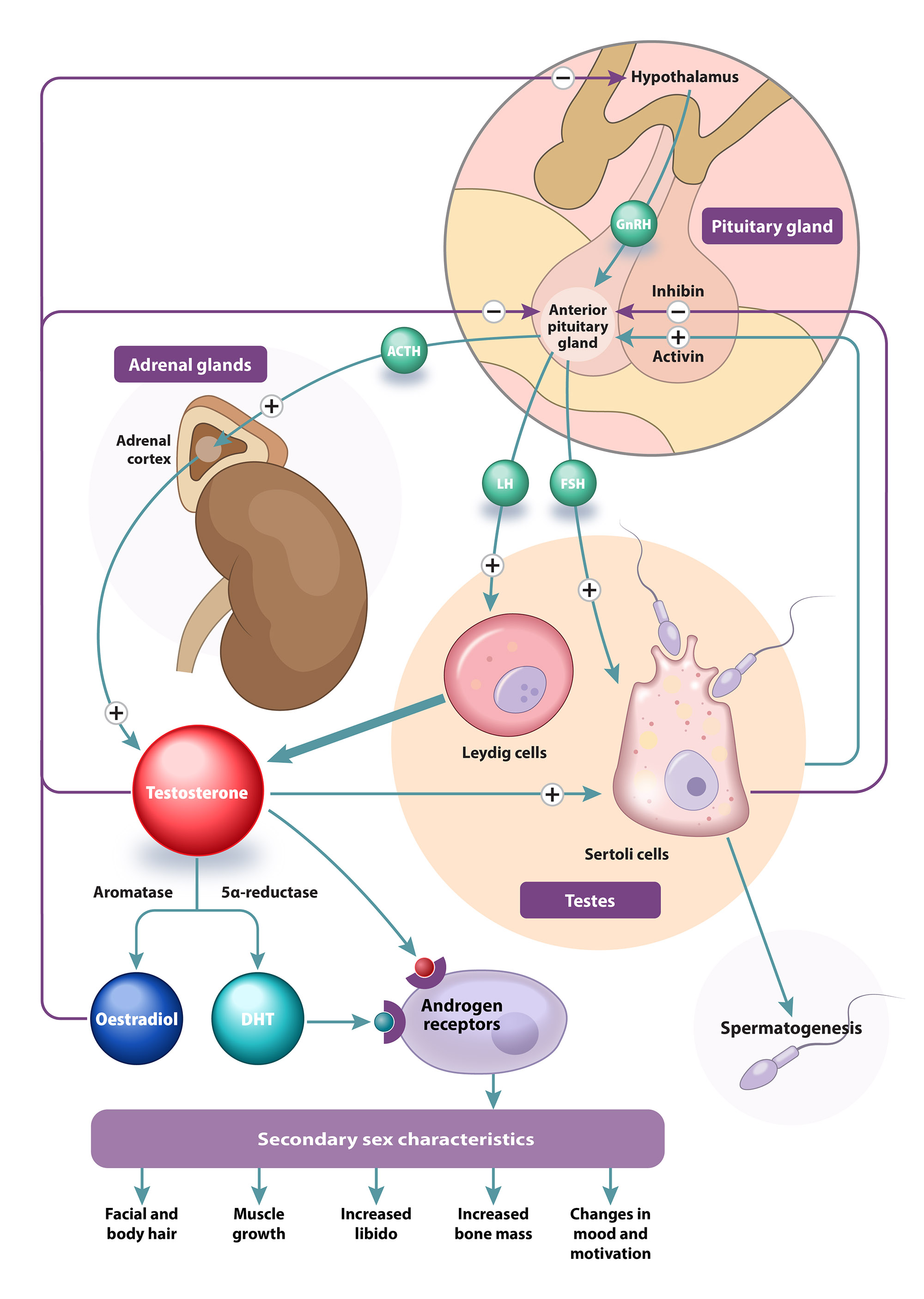
Figure 1. Production of testosterone is regulated by the hypothalamic-pituitary-gonadal axis.1, 2, 6
GnRH = gonadotropin-releasing hormone; ACTH = adrenocorticotropic hormone; LH = luteinising hormone; FSH = follicle-stimulating hormone; DHT = 5α-dihydrotestosterone
Testosterone levels decline with age
Serum testosterone generally declines very slowly in males, unlike the abrupt fall in oestrogen levels in females during menopause.2 Male total testosterone levels begin to gradually reduce from around age 35 years with an increasing rate of decline from approximately age 65 years.7 The diurnal rhythm (peak levels in the morning and lowest levels in the late afternoon/evening) also becomes less pronounced.7 SHBG concentrations in males increase with age, resulting in declining levels of free testosterone.7 A reduction in total and free testosterone is associated with an increased risk of developing type 2 diabetes and cardiovascular disease (CVD) morbidity and mortality.4, 5 However, it is unclear whether this relationship is causal; hypogonadism, diabetes and CVD risk are all influenced by common factors, e.g. obesity (particularly central adiposity), ageing.8 Severe hypogonadism (total testosterone < 3.5 nmol/L) can lead to osteopenia and osteoporosis, even in younger patients.4 Co-morbidities, lifestyle factors and the use of some medicines contribute to a reduction in testosterone levels as well (see: “Considerations when taking a patient history”).4, 7
Changes in reproductive hormone levels with age, however, are not inevitable or universal and some studies of healthy, active older males suggest they can retain levels comparable with younger males.7
Testosterone dispensing highest among males aged > 50 years in New Zealand
Approximately 0.3% of males in New Zealand were dispensed testosterone in 2023, and use is increasing each year.9 In 2023, 6,620 males were dispensed testosterone compared to 4,815 in 2018; a 37% increase.9 The largest group of males dispensed testosterone was aged between 50 and 69 years, but increases in dispensing rates occurred across all age groups during this period (Figure 2).9 Dispensing data does not provide the underlying reasons for prescription. This ongoing rise in the number of people using testosterone may be due to population growth, an ageing population, increasing clinical recognition and detection of hypogonadism, testosterone-based gender-affirming hormonal treatment, inappropriate prescribing, or any combination of these factors.
Rates of testosterone prescribing are rising most rapidly in males aged 20 to 39 years
Between 2018 and 2023, the number of males prescribed testosterone increased 120% among those aged 20 to 29 years and 80% among those aged 30 to 39 years.9 Possible explanations for this may include an increase in the number of transgender men (whose sex assigned at birth was female but have since had their health records updated to male) accessing testosterone-based gender-affirming hormonal treatment and social pressures on younger males regarding body image.

Figure 2. Total number of males dispensed testosterone (all formulations) by age group in New Zealand from 2018 to 2023.9 N.B. Figures are based on testosterone dispensed to patients recorded as male on their medical record; this may include medicines dispensed to people who were not recorded as male in their medical records at birth, i.e. testosterone-based gender-affirming hormonal treatment.
Diagnosis summary
- Patient history: does the patient report specific symptoms of hypogonadism? Could non-specific symptoms be explained by other causes, e.g. normal age-related changes, psychosocial factors?
- Clinical examination: are there any features that support a diagnosis of hypogonadism or suggest another possible cause?
- Investigation: does the patient have specific features of hypogonadism present, that are impacting their quality of their life, therefore warranting investigation of testosterone levels?
Values provided for diagnostic thresholds in this section are based on published position statements and international guidelines. Testosterone reference ranges vary significantly between laboratories and populations. A biochemical diagnosis of hypogonadism should be based on the reference range used by your local laboratory and the recommendations of local endocrinologists.
Sexual symptoms are most indicative for hypogonadism
The diagnosis of hypogonadism in older males can be challenging as many symptoms and signs (Table 2) are non-specific and may be associated with other conditions (e.g. obesity, type 2 diabetes, depression).10, 11 In some cases, psychosocial factors (e.g. stress, relationship issues), some medicines (e.g. antidepressants) or normal ageing could also explain some of these features.4 The presence of multiple, or more specific, features should increase diagnostic certainty. Studies have found that the presence of the following three sexual symptoms is likely to be an indicator of low testosterone levels:5, 12
- Decreased frequency of morning erections; AND
- Erectile dysfunction (inability to achieve or maintain penile erection for satisfactory sexual performance); AND
- Decreased frequency of sexual thoughts (low sexual desire)
Table 2. Clinical features and factors associated with hypogonadism.3–5, 13
| Symptoms and signs of testosterone deficiency in males |
| More specific |
Less specific |
Decreased or absent early morning/spontaneous erections
Erectile dysfunction
Reduced libido
Breast discomfort, gynaecomastia
Loss of facial, axillary and pubic hair
Testicular atrophy
Infertility, low sperm count
Height loss, low velocity fractures, low bone mineral density |
Decreased energy, motivation and confidence
Depressed mood
Irritability
Poor concentration and memory
Sleep disturbance and increased sleepiness
Mild anaemia (normochromic)
Reduced muscle bulk and strength
Increased body mass index and body fat
Hot flushes, sweats |
| Factors associated with hypogonadism |
| Medical conditions |
Medicines and medical treatments |
Lifestyle factors |
End-stage renal disease
Liver disease, e.g. cirrhosis
Osteoporosis
Moderate to severe COPD
Post-acute COVID-19 syndrome (“long COVID”)
Type 2 diabetes
Insulin resistance/metabolic syndrome
Depression
Pituitary tumour
HIV
Testicular cancer
Haemochromatosis
Chronic inflammatory disease
Malignancy
Eating disorders (malnutrition/nutritional deficiency) |
Opioids
High-dose systemic corticosteroids, e.g. prednisone
Anabolic steroids
Antipsychotics, e.g. risperidone
Anticonvulsants, e.g. carbamazepine, sodium valproate
Oestrogens and progestogens
5α-reductase inhibitors, e.g. finasteride
Chemotherapy medicines
Radiation treatment |
Obesity
Chronic excess alcohol intake (recent alcohol intake can cause a transient decrease in total testosterone levels)
Excessive exercise
Significant stress
Sleep deprivation
Illicit drug use (including misuse of anabolic steroids) |
COPD = chronic obstructive pulmonary disease; COVID-19 = SARS-CoV-2 coronavirus; HIV = human immunodeficiency virus
Considerations when taking a patient history
A number of medical conditions and medicines can influence the function of the hypothalamic-pituitary-gonadal (HPG) axis and are associated with hypogonadism (Table 2).3 Patients with these conditions or receiving these treatments do not necessarily need to be investigated for hypogonadism, but their presence, along with symptoms and signs of testosterone deficiency, would increase clinical suspicion. N.B. Some medicines may be indirectly associated with symptoms of hypogonadism, e.g. spironolactone causing antiandrogenic effects, selective serotonin reuptake inhibitors (SSRIs) causing sexual dysfunction.
Assess for mental health conditions and ask about psychosocial factors that may contribute to symptoms. Observational studies have demonstrated an association between low testosterone levels and depression; many non-specific symptoms associated with hypogonadism are also reported in people with major depressive disorder, e.g. low mood, sleep disturbance, memory impairment.4, 14 Erectile dysfunction is frequently reported in people experiencing depression or anxiety-related disorders.14 Life stressors and relationship issues also contribute to sexual dysfunction.4 If identified, address these before progressing.
The patient’s lifestyle can affect testosterone levels. For example high volume endurance exercise and sleep restriction reduce serum testosterone in males.15, 16
Consider a pituitary cause in patients who present with headaches or visual disturbances in addition to conventional symptoms of hypogonadism,4 as well as other suspicious findings, e.g. unexplained low cortisol or free T4 with normal/low TSH.
Ask patients about testosterone supplementation. Evaluating the HPG axis is difficult in patients with current or recent testosterone use due to suppression of endogenous testosterone production, e.g. anabolic steroids, selective androgen receptor modulators (SARMs) or prescribed testosterone supplementation. These effects may persist for weeks to months after stopping treatment. N.B. Evidence supporting most over-the-counter herbal products marketed as “testosterone boosters” is weak and they are unlikely to affect serum testosterone levels, however, supplements from unregulated sources may be contaminated or contain prescription medicines (i.e. testosterone).17
Clinical examination:3, 5
- Assess body hair distribution, i.e. hair loss compared to previous pattern
- Assess testicular size and any changes
- Assess breast tissue and any changes
- Measure height, weight, waist circumference and calculate body mass index (BMI)
- Perform a digital rectal exam to assess prostate size and to exclude nodules or indurations (contraindication to testosterone treatment; see: “Potential risks of testosterone replacement treatment”)4
Investigate testosterone levels only if significant features of hypogonadism are present
A diagnosis of hypogonadism in males can be made based on the presence of symptoms and signs of testosterone deficiency in combination with a finding of low testosterone levels.4, 5 Investigate other potential causes of declining strength, reduced muscle mass or body composition in the absence of sexual symptoms and address co-morbidities before requesting a testosterone measurement. Measuring testosterone is not routinely required in asymptomatic patients.4
Serum total testosterone should be measured between 7 am and 11 am, ideally after an overnight fast (Figure 3).4, 5 Acute illness, recent food (carbohydrates) or alcohol intake may cause a transitory drop in testosterone levels.5, 13
Repeat the test for confirmation if the patient’s testosterone level is below the reference range (preferably four weeks later); a repeat test is not required if the level is normal.3 Luteinising hormone (LH), follicle-stimulating hormone (FSH) and prolactin levels should be requested as part of confirmatory testing.3 Prolactin is ideally measured in the afternoon and circulating levels are increased by certain medicines (e.g. tricyclic antidepressants, benzodiazepines, anti-psychotics and SSRIs); a 48 hour washout period is recommended but may not be practical in all cases.13
Discuss the need for further testing with an endocrinologist or the laboratory. This may include:
- SHBG (to calculate free testosterone) – not routinely recommended but may be useful to confirm a diagnosis of hypogonadism in patients with borderline total testosterone levels or with clinical factors that increase SHBG (see: “Measuring free testosterone for confirmation”)
- Thyroid stimulating hormone (TSH), thyroxine (T4), insulin-like growth factor-1 (IGF-1) and morning plasma cortisol – to identify wider endocrine dysfunction if clinical examination indicates a possible pituitary cause10, 11

Figure 3. A suggested approach to the investigation of serum testosterone levels and treatment initiation in patients with clinical features of hypogonadism.3–5 N.B. Reference ranges for testosterone differ depending on the immunoassay used; consult with your local laboratory.
* Measure total testosterone in a shift worker two to three hours after waking
FSH = follicle-stimulating hormone; fT = free testosterone; IGF-1 = insulin-like growth factor-1; LH = luteinising hormone; SHBG = sex hormone-binding globulin; TSH = thyroid-stimulating hormone; tT = total testosterone; T4 = thyroxine
The threshold testosterone level for hypogonadism is unclear
The testosterone level at which symptoms and signs of deficiency become apparent varies between individuals, but the likelihood of symptoms begins to increase with testosterone levels < 12 nmol/L, and rises substantially below approximately 8 nmol/L.3, 12 International guidelines differ on the threshold for diagnosis of hypogonadism, e.g. 12 nmol/L total testosterone in the European Association of Urology (EAU) guidelines, compared with 8 nmol/L in the European Menopause and Andropause Society (EMAS) guidelines.4, 5 Local expert opinion is that the threshold for hypogonadism diagnosis and initiating testosterone treatment should be low, i.e. < 8 nmol/L, as older patients often have circulating levels < 12 nmol/L but do not require testosterone replacement treatment.
Consider discussing patients with consistently low testosterone levels (i.e. two morning serum testosterone levels between 8 and 12 nmol/L) or inconclusive testosterone levels with an endocrinologist, especially if guidance is required regarding further investigations or appropriate management (see: “Managing older males with established testosterone deficiency”). N.B. Measuring testosterone levels in asymptomatic patients is not recommended; however, if a test was done and the testosterone level was < 6 nmol/L, replacement treatment could be considered in an asymptomatic patient. Discussion with an endocrinologist is advised.
Measuring free testosterone for confirmation
Diagnosis of hypogonadism by directly measuring free testosterone is not recommended due to the unreliability of available immunoassays.3, 5 Indirect measurement of free testosterone (calculated from total testosterone and SHBG levels using the Vermeulen equation) is preferred to confirm hypogonadism if diagnostic uncertainty remains following two morning serum testosterone levels.5 Some males with total testosterone levels in the lower normal range may exhibit hypogonadism due to elevated SHBG levels and low free testosterone (reduced bioavailability).3 A free testosterone level < 0.220 nmol/L (< 220 pmol/L) in patients with relevant symptoms is likely indicative of hypogonadism,4 whereas a normal free testosterone level generally excludes testosterone deficiency (even if the total testosterone is below the reference range). Indirect measurement of free testosterone may also be appropriate in patients with clinical features that affect SHBG levels (Table 1).4 Discuss clinical requirements for free testosterone testing with your local laboratory beforehand as there may be restrictions in some regions, e.g. testing only funded if total testosterone measurement is inconclusive (i.e. 7 – 15 nmol/L) or endocrinologist recommendation required.
Classifying hypogonadism
Primary hypogonadism (testicular dysfunction) is characterised by low testosterone levels in conjunction with elevated LH levels.3 FSH may be elevated if seminiferous tubule function and spermatogenesis is also affected.13 Secondary hypogonadism (pituitary dysfunction) is commonly associated with low LH or inappropriately normal levels of LH in a patient with low testosterone levels.3, 5
Clinical suspicion of a pituitary tumour should be increased in patients with biochemically unequivocal secondary hypogonadism (e.g. total testosterone < 8 nmol/L, especially < 5 nmol/L, and low LH and FSH) and evidence of deficiency of other endocrine axes, e.g. elevated prolactin, low T4 with normal or low TSH, low IGF-1.3, 10 Refer for endocrinology assessment in this situation.
Erectile dysfunction is a common reason for men to request testosterone investigation
Investigation of testosterone levels is usually unnecessary in patients presenting with erectile dysfunction and no other symptoms of hypogonadism. Potential causes of erectile dysfunction are diverse, and include neurological or vascular disease, medicines or psychological factors; hormonal disturbances are only a factor in some cases.1 Most males with erectile dysfunction do not have low testosterone levels.1 In addition, testosterone monotherapy is not an effective management strategy for patients with moderate to severe erectile dysfunction.1, 4 A phosphodiesterase type-5 inhibitor, such as sildenafil, is the first-line pharmacological treatment, after modifiable causes have been addressed.18
It is reasonable to request a total testosterone level in patients presenting with erectile dysfunction along with other symptoms of hypogonadism (e.g. decreased frequency of morning erections, low libido). However, be aware that testosterone treatment may worsen the patient’s situation if hypogonadism is not the underlying cause, e.g. erectile dysfunction and performance anxiety causing low libido. Testosterone could also be investigated in patients who have had an unsatisfactory response to a phosphodiesterase type-5 inhibitor trial or patients with erectile dysfunction and type 2 diabetes.18
Testosterone replacement treatment can be discussed as an option to improve sexual dysfunction symptoms if a testosterone deficiency is established.3 Improvements in libido and sexual function in general have been reported in males taking testosterone replacement.4 A positive effect on erectile dysfunction symptoms with testosterone replacement is more likely in males with lower baseline testosterone levels, e.g. < 8 nmol/L.4 However, testosterone treatment may also have a significant placebo effect in this scenario. Consider this possibility when deciding whether continuing testosterone treatment is appropriate in patients whose symptoms return after a period of initial improvement (e.g. six months). There is limited evidence directly comparing testosterone treatment against phosphodiesterase type-5 inhibitors in the management of erectile dysfunction, however, when used in combination, it is unlikely that that the addition of testosterone treatment is significantly more effective than a phosphodiesterase type-5 inhibitor and placebo.19
Management summary
- Address modifiable factors: does the patient have co-morbidities (including the effect of medicines) or lifestyle factors that can be optimally managed to improve symptoms?
- Consider testosterone treatment: does the patient have specific features for which improvement with testosterone treatment can be measured/assessed? Does the patient have any contraindications or cautions? Have the benefits and risks of treatment and monitoring requirements been explained to the patient?
- Second opinion: Can testosterone replacement treatment be confidently initiated? Or should the management strategy and possible treatment options be discussed with a more experienced colleague or an endocrinologist first?
- Monitoring: have symptoms improved with treatment? Are there any adverse effects?
Address reversible causes of hypogonadism first
If a patient is confirmed to have hypogonadism, optimise management of any co-morbidities, medicines or lifestyle factors that may be contributing to their clinical condition (Table 2) before considering testosterone replacement treatment.4 In some situations, switching, reducing or deprescribing medicines associated with hypogonadism may be required, e.g. psychotropics such as opioids or antipsychotics, corticosteroids. Recommend non-pharmacological interventions and lifestyle changes, if appropriate, e.g. increasing physical activity, weight loss, smoking cessation, reducing excessive alcohol consumption, stress reduction.
Discuss the benefits and risks of testosterone replacement treatment
Testosterone treatment in older males should only be considered in those with clinically established hypogonadism and with features that significantly impact their quality of life, and for which improvements can be evaluated. Evidence does not support the use of testosterone to improve cardio-metabolic health, physical strength, mobility or body composition, in ageing males without established pathological hypogonadism; prescribing testosterone in this situation is not recommended.4 Potential adverse effects, monitoring requirements, a lack of conclusive evidence regarding long-term risks and the need for life-long treatment (if beneficial) should be discussed with the patient to help them decide if they want to proceed with testosterone treatment.
Is testosterone use in older males safe? The jury is still out.
Much of the concern regarding testosterone replacement treatment in ageing males relates to CVD risk. Between 2010 and 2014, a small number of studies investigating testosterone use in older males found evidence of increased CVD risk, generating widespread media coverage.20–22 At the time, organisations and committees such as the United States’ Food and Drug Administration (FDA) and American Urological Association (AUA) came to differing conclusions regarding the risk for males prescribed testosterone, reflecting the recognised weaknesses in study design and varying interpretations of the evidence. In 2014, New Zealand’s Medicines Adverse Reactions Committee (MARC) determined there was “no significant statistical evidence to support an association between testosterone therapy with myocardial infarction, venous thromboembolism, or stroke,” based on the available research at the time.23
The TRAVERSE trial was expected to provide answers
The Testosterone Replacement Therapy for Assessment of Long-term Vascular Events and Efficacy Response in Hypogonadal Men (TRAVERSE) trial was a randomised, double-blinded, placebo-controlled study undertaken to assess the cardiovascular safety of testosterone replacement treatment. The study involved 5,204 males aged 45 to 80 years (mean age 63 years) with symptomatic hypogonadism, two morning fasting testosterone levels < 10.4 nmol/L, and either pre-existing CVD or at elevated CVD risk.24 The intervention group received daily transdermal testosterone (1.62%) gel and the control group received a placebo gel.2 The study was anticipated to run for five years, but was ended after 22 months as there was no clear difference between intervention and control groups.24, 25
Testosterone was shown to be non-inferior to placebo for the occurrence of major adverse cardiovascular events.24 There was no increase overall or in any specific secondary CVD outcome events (MI, stroke, cardiovascular death) in participants receiving testosterone compared to placebo.24 However, higher rates of pulmonary embolism (treatment group: 0.9% vs control group: 0.5%), non-fatal arrhythmias (5.2 vs 3.3%), atrial fibrillation (3.5 vs 2.4%) and acute kidney injury (2.3 vs 1.5%) were reported in the intervention group.24 A higher fracture rate was also observed in the intervention group (3.5 vs 2.5%).26 The study had several limitations including participants in the intervention group only just reaching target testosterone levels (median total testosterone of 12.9 nmol/L in the intervention group after 12 months compared to a target range of 12.1 – 26.0 nmol/L), a high discontinuation rate during follow-up, increased participant drop out compared to comparative studies and funding from pharmaceutical companies that manufacture testosterone products.24, 27
What can we take from this? Caution is still advised.
The overall risk of adverse cardiovascular effects does not appear to be further increased in older males treated with testosterone for fewer than three years.4 However, a cautious approach to prescribing is still warranted due to the possible increased risk of specific CVD morbidities. The shortened duration of the TRAVERSE trial also means further investigation is needed to provide long-term safety information (i.e. longer than three years) in this patient group given that testosterone treatment is often used life-long.4, 5
Benefits of testosterone replacement treatment
Treatment response varies between patients but may include a reduction in mild depressive symptoms and improvements in bone mineral density, as well as reported improvements in libido and sexual function.4 For males with erectile dysfunction, evidence suggests that a phosphodiesterase type-5 inhibitor (e.g. sildenafil) alone is more effective for improving erectile function in the short-term compared to the addition of testosterone (see: “Erectile dysfunction is a common reason for men to request testosterone investigation”).19 Other potential benefits of testosterone replacement include improvement in body composition (e.g. increased muscle mass, decreased abdominal fat) and surrogate markers of CVD risk (e.g. improved glycaemic control and lipid profile), however, evidence is inconsistent.3, 4
Potential risks of testosterone replacement treatment
Secondary polycythaemia is the main adverse effect of testosterone treatment, detected by an elevation in haematocrit levels (packed cell volume; PCV), often occurring between 3 – 12 months after starting treatment.4 It is caused when levels of testosterone rise above the normal physiological range and is more common with injectable formulations where the treatment regimen causes fluctuating testosterone levels.10 Polycythaemia can lead to cardiovascular and thrombotic complications due to the increased viscosity of the blood, e.g. venous thromboembolism.5 Testosterone should be used with caution in males with pre-existing CVD (see: “Is testosterone use in older males safe? the jury is still out.”).4
Prostate volume may increase with testosterone treatment,5 leading to lower urinary tract symptoms (LUTS). However, testosterone treatment has not been shown to worsen LUTS in males who already experience mild symptoms.4, 5 Use testosterone with caution in males with severe LUTS as they are often excluded from clinical trials.4, 5
Prostate cancer risk in the long-term is unclear. Short-term testosterone replacement treatment (i.e. approximately three years or less) is not associated with an increased risk of prostate cancer based on current evidence,4, 28 however, testosterone is often used life-long. Discuss the absence of long-term safety data regarding prostate cancer with patients before initiating treatment.4 Testosterone may also increase the rate of growth of pre-existing androgen-dependent prostate cancer cells,29 highlighting the importance of endocrinology involvement in the management of patients with prostate cancer risk factors.
Sperm production is reduced via negative feedback on the HPG axis; testosterone is contraindicated in males who wish to remain fertile.3, 4 Depending on the underlying cause, suppressed spermatogenesis due to exogenous testosterone can be reinitiated by human chorionic gonadotropin (hCG) treatment,3 usually at a private fertility clinic. Warn patients that restoration of fertility is dependent on their age, dose and duration of testosterone treatment and testicular function before initiating treatment.30 Restoration of spermatogenesis can take 12 months or more following treatment cessation, and may not be possible in some cases.3, 30
Changes in mood and behaviour have been reported in patients taking testosterone, especially in males with impulsive or dominant personality types.5 Discuss the potential for aggressive behaviour and mood swings before initiating treatment. A discussion between the patient and their partner/family/whānau may also be beneficial. Advise patients to return for evaluation if this occurs.
Other adverse effects experienced by males using testosterone include acne, worsening male pattern baldness, injection site reactions (injectable formulations) and skin irritation (transdermal formulations).3, 10 Oral formulations have been associated with small increases in systolic blood pressure, i.e. 3 – 5 mmHg, and may not be appropriate for older people with hypertension.5
Testosterone replacement treatment is contraindicated in males with:3, 4, 31
- Metastatic prostate cancer (or palpable prostate nodule or induration)
- Prostate-specific antigen (PSA) > 4.0 ng/mL, or > 3.0 ng/mL if family history of prostate cancer
- History of liver tumours
- History of breast cancer
- Haematocrit (PCV) ≥ 54%
- Hypercalcaemia
- Requirement for fertility
- Severe or poorly controlled congestive heart failure
Use testosterone replacement treatment with caution in males with:4, 31
- Cardiac, renal or hepatic disease
- History of localised prostate cancer
- Significant LUTS associated with benign prostatic hyperplasia (BPH)
- Haematocrit in the upper limit of normal, i.e. 48 – 50%
- Personal or familial history of venous thromboembolism or risk factors
- Hypertension
- Epilepsy
- History of migraine
- Diabetes
Obstructive sleep apnoea no longer a contraindication
Obstructive sleep apnoea has previously been a contraindication to testosterone replacement treatment, however, this was based on weak evidence.4, 32 A 2012 study involving 54 participants reported that compared to placebo, obese males receiving testosterone showed worsening markers of sleep apnoea after seven weeks, e.g. nocturnal hypoxaemia, oxygen desaturation events.33 This effect was not associated with baseline testosterone levels and disappeared after 18 weeks of treatment.33 The most recent EAU guidelines concluded that testosterone replacement treatment does not induce or worsen obstructive sleep apnoea and removed it as contraindication.4
Complete a full investigation for an organic cause of hypogonadism before initiating treatment as testosterone replacement makes identifying the underlying cause difficult. Baseline testing before commencing testosterone replacement treatment is also essential, as detailed in Table 4. Only initiate testosterone treatment in symptomatic patients with laboratory-confirmed hypogonadism on two separate occasions.
If testosterone treatment is clearly indicated and the patient wishes to proceed, primary care clinicians may feel confident enough to initiate treatment. However, discussion with an endocrinologist is recommended if the prescriber is uncertain whether to proceed or has limited experience initiating testosterone in patients with hypogonadism.
A six-month trial of testosterone replacement treatment is recommended to evaluate clinical response and adverse effects.3 Advise patients that beneficial effects may not be apparent for at least three months and the maximal therapeutic effect often requires 12 months of treatment.3, 4 Patients who do experience symptom improvement will likely require testosterone treatment long-term.3
Prescribe testosterone gel initially
Transdermal gel is preferred for initiating testosterone treatment as daily dosing allows for dose adjustment or withdrawal if adverse effects develop;4 see Table 3 for dosing and application instructions. Switching to a different formulation may be considered once the testosterone dose has been stabilised and evidence of benefit is established. Intramuscular testosterone injections are fully funded, but oral capsules (Section 29) are subsidised by endorsement only for patients prescribed them prior to 1st November, 2021, therefore, new patients cannot be initiated on them.31 Testosterone cream and a transdermal testosterone implant are also available in New Zealand but are not funded.31 Testosterone patches were previously funded, however, these were discontinued and removed from the Pharmaceutical Schedule in November, 2024.
Table 3. Funded testosterone formulations available in New Zealand.4, 31, 34
| Formulation |
Strength |
Dosing |
Notes |
| Transdermal applications |
| Transdermal testosterone gel |
16.2 mg/g |
Initially 40.5 mg (approximately two pump actuations), once daily
Adjust dose in increments of 20.25 mg (approximately one pump actuation) based on clinical response; maximum dose 81 mg in 24 hours |
Apply a thin layer to dry skin on upper arms and shoulders at the same time each day (ideally in the morning). Do not apply to genitals.
Allow to dry for 3 – 5 minutes before getting dressed
Wash hands after application. Also, wash application site prior to skin-to-skin contact with another person to avoid inadvertent transfer.
Delay bathing/showering by one hour after applying gel |
| Intramuscular (IM) injections |
| Testosterone esters |
250 mg/mL |
250 mg, every three weeks |
Fluctuations in testosterone levels occur across the injection cycle with higher levels following injections, tapering off over time. Patients may experience corresponding fluctuations in symptoms of hypogonadism.
Risk of elevated haematocrit (and other cardiovascular adverse effects) is greater with injections compared to other testosterone formulations due to periods of elevation of testosterone levels above physiological range |
| Depo-testosterone (testosterone cipionate) |
1 g/10 mL |
50 – 400 mg, every two to four weeks |
| Testosterone undecanoate (undecylate) |
1 g/4 mL |
1 g, every 10 to 14 weeks
Administer slowly to reduce injection site pain |
Provides stable testosterone levels over time compared to other IM testosterone injections
Cannot be withdrawn if adverse effects develop
Accounted for more than 40% of dispensed testosterone in New Zealand in 20239 |
N.B. Testosterone undecanoate oral capsules (existing patients only) and transdermal testosterone patches (discontinued) are not included in this table (see text).
Regular monitoring is required during testosterone treatment
Patients should be followed up at 3, 6 and 12 months and then annually, thereafter.4 Evaluate the patient’s clinical response and address any adverse effects.4 See Table 4 for specific monitoring recommendations.
Testosterone – The ideal target testosterone level in ageing males is unknown.5 International guidelines typically advise maintaining circulating levels within the mid-normal physiological range for the patient’s age,3, 4 whereas local expert opinion is to maintain testosterone levels in the lower half of the normal reference range (see local laboratory for specific reference intervals as these differ). In practice, the target testosterone level is dependent on treatment response and patient age. Gradual titration to a level where symptom improvement occurs is appropriate, however, high-normal levels should be avoided in older patients.
The optimum time for monitoring testosterone levels depends on the formulation:3, 4, 32
- Transdermal gel – measure two to eight hours after application
- Injectable formulations – measure midway between the planned dosing interval
- Injectable testosterone undecanoate – measure at the end of the dosing interval
- Oral testosterone undecanoate capsules – measure within three to five hours of dosing, however, absorption is unpredictable and peak and trough levels vary substantially
- Transdermal patches – measure 3 – 12 hours after application, e.g. in the morning if the patch was applied the previous evening
PSA – Small increases in PSA levels may occur after the first three months of treatment but should stabilise within normal levels after 12 months; larger increases > 1 ng/mL are uncommon.5 Discussion with, or referral to, a urologist is recommended in patients with a PSA increase > 1.4 ng/mL over a 12-month period following initiation of testosterone replacement treatment, if PSA velocity is > 0.4 ng/mL/year (if taking testosterone for more than two years, and preferably from multiple measurements per year spaced three to six months apart), if prostatic symptoms develop or if a prostatic abnormality is detected.3, 13
For further information on PSA testing in primary care, see: https://bpac.org.nz/2020/prostate.aspx
Full blood count – Stop testosterone in patients with CVD risk factors whose haematocrit increases > 54%.4 Reinitiation of testosterone at a lower dose or with a different formulation may be possible once haematocrit levels return to normal.4 In those with a low CVD risk, an increase in haematocrit levels may not require treatment cessation; consider reducing the dose or switching the formulation.4
There are no specific monitoring requirements for cardiovascular health, however, most older males receiving treatment for hypogonadism will already be undergoing regular CVD risk assessments. Advise patients to seek prompt medical attention if new cardiac symptoms develop.
Discontinue treatment if adverse effects are intolerable or severe, or if no clinical benefit is observed within six months.3 Tapering is not required when discontinuing testosterone.35
Table 4. Initial and ongoing monitoring recommendations for patients taking testosterone replacement treatment.3–5
| Investigation |
Baseline |
3 months |
6 months |
12 months |
Every 12 months thereafter |
Alternative monitoring schedule |
| Testosterone |
 |
 |
 |
 |
 |
|
| PSA* |
 |
 |
|
 |
 |
Or according to local screening guidelines |
| DRE |
 |
|
|
 |
 |
Performed earlier if a significant rise in PSA velocity |
Full blood count
(haemoglobin and haematocrit) |
 |
 |
 |
 |
 |
|
| HbA1c |
 |
|
|
 |
 |
|
| Lipids |
 |
|
|
 |
 |
|
| Blood pressure |
 |
 |
|
 |
 |
|
| BMI |
 |
|
|
 |
 |
|
| Waist circumference |
 |
 |
|
 |
 |
|
DEXA scan
(cost associated in some regions) |
Consider in patients with severe hypogonadism |
|
|
|
|
Between 18 months and 5 years after initiating treatment depending on patient’s fracture risk |
BMI = body mass index; DEXA = dual-energy X-ray absorptiometry; DRE = digital rectal examination; PSA = prostate-specific antigen
* Measure PSA in patients who have previously undergone treatment for prostate cancer after 3, 6 and 12 months, and then annually





