Published: 28 April 2023 | Updated: 11 March 2024 | What's changed?
11 March 2024 Cervical screening recommendations after treatment for cervical HSIL
updated to align with the HPV Primary Screening programme
Key practice points:
- The vulva is affected by the same types of cancer that affect skin elsewhere, including squamous cell carcinoma (SCC), basal cell carcinoma and melanoma
- SCC accounts for approximately 90% of all vulval cancers. It is usually preceded by vulval intraepithelial neoplasia (VIN), either differentiated VIN or high-grade squamous intraepithelial lesions (HSIL).
- Vulval SCC develops through two pathways:
- Chronic vulval inflammation, e.g. due to lichen sclerosus or erosive lichen planus, with vulval SCC usually preceded by differentiated VIN; referred to as the human papillomavirus (HPV)-independent pathway
- Infection with high-risk HPV types, with vulval SCC usually preceded by HSIL; referred to as the HPV-associated pathway. HSIL are more common than differentiated VIN, but are less likely to progress to invasive vulval SCC.
- Historically, most vulval SCC arose from the HPV-independent pathway, but the epidemiology has now changed in New Zealand and the proportions of vulval SCC from both pathways are almost equal
- Additional risk factors for vulval cancer include increasing age, cigarette smoking and immune deficiency
- The risk of developing VIN (HSIL or differentiated VIN) and therefore, squamous cell vulval cancer can be reduced through prophylactic HPV vaccination with Gardasil 9 (currently recommended vaccine; prevents new infection with HPV) and good control of inflammatory conditions such as lichen sclerosus
- In most cases, vulval cancer is diagnosed in females who are post-menopausal, although HPV-associated vulval cancers generally occur 10 – 15 years earlier than HPV-independent vulval SCC
- Symptoms and signs of vulval cancer may include vulval pruritus, pain, bleeding, discharge or a vulval lesion or mass. Patients can also have a lesion but be asymptomatic.
- There are no screening programmes for the early detection of vulval cancer, but some people are diagnosed incidentally after an examination for other reasons, e.g. cervical screening. Most people are diagnosed from targeted investigations after symptoms are reported.
- The diagnostic workup of a patient with suspected vulval cancer includes a focused history, considering relevant risk factors and a vulval examination with particular attention to the labia majora as this is the most common site of vulval cancer
- Vulval cancers can vary widely in appearance and may be multifocal or involve a single lesion. Features of malignancy include: irregular epithelial surfaces, swelling, red, white or other pigmented areas, visible lesion/mass, ulceration, bleeding.
- Patients with vulval symptoms but no visible lesion are unlikely to have vulval cancer
- A biopsy is necessary for the diagnosis of VIN and vulval cancer. Refer patients with abnormal examination findings, e.g. suspicious vulval lesion, to a gynaecologist for further assessment.
N.B. The term “female” is used in this article to describe the biological sex of the patient population at risk for vulval cancer. However, we acknowledge that this may not reflect the identity of all patients, which will include transgender boys or men, intersex and non-binary individuals.
For information on the follow-up and surveillance of a patient after curative-intent treatment for vulval cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
Vulval cancer accounts for approximately 5% of all gynaecological cancers in New Zealand with an average of 52 new diagnoses (1.4 per 100,000 females; from 2015 – 2020) and 16 deaths (0.4 per 100,000 females; from 2015 – 2018*) each year;† fewer than all other gynaecological cancers except vaginal cancer.1 Squamous cell carcinoma (SCC) is the most common type of vulval cancer (approximately 90% of cases), and is usually associated with lichen sclerosus or high-risk human papillomavirus (HPV) infection.2
*Mortality data are available for 2019, but are preliminary so have not been included. Mortality data for 2020 are not yet available.
†There are differences in published data. For consistency across the gynaecological cancer series, incidence and mortality data has primarily been obtained from the publication – Cancer: Historical summary 1948 – 2020.
There is no screening test for vulval cancer, so diagnosis relies on investigating reported vulval lesions or symptoms, opportunistic detection of vulval abnormalities, e.g. during cervical screening, and following up patients who are at increased risk, e.g. those with lichen sclerosus.3
The risk of vulval cancer increases with age and it primarily affects females who are post-menopausal with most diagnoses occurring in those aged > 70 years.2 Vulval cancer can, however, still occur in younger females; internationally the incidence in those aged < 60 years has increased since the early 1990s, likely due to an increase in the prevalence of HPV infection in younger age groups.2 In New Zealand, the incidence of vulval cancer associated with high-risk HPV infection is increasing in females aged > 50 years.4
The prognosis of females with vulval cancer is usually good if diagnosed early. Overall five-year survival for people with vulval SCC is 71%;5 this can be above 80% in those with no lymph node involvement.3 However, five-year survival is below 50% for people with spread to the inguinal lymph nodes and 10 – 15% if the iliac or other pelvic nodes are involved.3 People with HPV-independent vulval SCC have a significantly poorer prognosis (25% worse) than those with HPV-associated vulval SCC.6, 7 This is independent of age at diagnosis and cancer stage, with poorer outcomes and risk of recurrence even at an early stage.6, 7
Risk factors for vulval cancer
The most significant risk factors for vulval SCC are chronic inflammatory conditions of the vulva, e.g. lichen sclerosus, erosive lichen planus, and infection with high-risk HPV, particularly type 16 (see: “Prophylactic HPV vaccination can reduce the risk of vulval cancer”).2 Estimates of lifetime risk of vulval cancer in people with lichen sclerosus range from 2.6 – 6.6%.2 For information on the treatment and follow-up of a patient with lichen sclerosus, see: “Lichen sclerosus”.
Additional risk factors include Paget disease of the vulva, smoking and increasing age; factors that increase the likelihood of HPV infection (e.g. high lifetime number of sexual partners, young age at sexual activity onset, immune deficiency) also therefore increase the risk of vulval cancer.5, 8, 9
Prophylactic HPV vaccination can reduce the risk of vulval cancer
Persistent infection with high-risk HPV types (particularly type 16) is associated with the development of high-grade squamous intraepithelial lesions (HSIL) and vulval cancer.2, 10 In New Zealand, HSIL makes up 95% of vulval cancer precursor lesions.11 Prophylactic HPV vaccination prevents new infection with HPV, including high-risk type 16, and therefore protects against the development of HPV-associated pre-cancerous vulval lesions.2, 10 It does not reduce the progression of established vulval lesions or cancer.10
HPV vaccination is most effective when administered prior to HPV exposure, however, it is still effective after exposure to HPV (see: “Expert tip”), and can prevent the development of up to 100% of pre-cancerous vulval lesions and vulval cancers.12, 13 There is also emerging evidence that HPV vaccination may be beneficial in reducing recurrence risk after treatment, however, further studies are required.14
Gardasil 9 is the currently recommended vaccine in New Zealand and has been used since 2017. It protects against nine types of HPV (6, 11, 16, 18, 31, 33, 45, 52, 58); seven of which cause HPV-related cancers and two cause genital warts (6, 11).10 HPV types 16, 18, 31, 33, 39 and 45 are responsible for the majority of HPV-associated vulval squamous cell carcinomas.11
 HPV vaccination is recommended for all females (and males) ideally before the onset of sexual activity, and is funded for eligible people aged 9 – 26 years inclusive*.10 School immunisation programmes and general practices generally offer HPV vaccination to students in Year Eight (around age 12 years).
HPV vaccination is recommended for all females (and males) ideally before the onset of sexual activity, and is funded for eligible people aged 9 – 26 years inclusive*.10 School immunisation programmes and general practices generally offer HPV vaccination to students in Year Eight (around age 12 years).
Expert tip.Vaccinating people who have already commenced sexual activity is still recommended as even if they have been infected with one or several HPV types, there are still other types of HPV that are associated with malignancy, and so it is unlikely that someone will have been infected with all of them.
N.B. Gardasil 9 is registered for use in females aged 9 – 45 years and in males aged 9 – 26 years. However, there are no theoretical concerns that the efficacy or safety of the vaccine in males aged up to 45 years will differ significantly from females of the same age or younger males.10 The vaccine may have efficacy in people aged > 45 years, however, there is a lack of evidence of this.
*If the course is started prior to the patients 27th birthday, the rest of the course is funded. For further information on funded indications, see: www.health.govt.nz/publication/immunisation-handbook-2020
For further information on HPV vaccination, see: “Cervical cancer – early detection and referral”
Approximately 90% of all vulval cancers are of SCC histology, and are generally preceded by precursor lesions, either differentiated VIN or HSIL (see Table 1 for explanation of terminology).2, 8, 15 The remaining 10% of vulval cancers predominantly include melanoma, Bartholin gland carcinoma, basal cell carcinoma and Paget disease of the vulva (see: “Rare types of vulval cancer”).2
Table 1. Terminology used to describe vulval intraepithelial neoplasia (VIN).
|
Current terminology* |
Previous terminology |
Association with vulval cancer
(also see Figure 1) |
| Vulval intraepithelial neoplasia (VIN) |
| High-grade squamous intraepithelial lesions (HSIL) |
Usual-type VIN (uVIN)
VIN 2 or 3
Bowen disease of the vulva |
HPV-associated; may progress to vulval SCC |
| Differentiated vulval intraepithelial neoplasia (differentiated or dVIN) |
No change |
HPV-independent; more likely to progress to vulval SCC |
| Low-grade squamous intraepithelial lesions (LSIL) |
Flat condyloma
HPV effect
VIN 1 |
HPV-associated; does not require treatment |
*Introduced in 2015 by the International Society for the Study of Vulvovaginal Disease: Bornstein J, Bogliatto F, Haefner HK, et al. The 2015 International Society for the Study of Vulvovaginal Disease (ISSVD) Terminology of Vulvar Squamous Intraepithelial Lesions. Obstetrics & Gynecology 2016;127:264–8. doi:10.1097/AOG.0000000000001285.
Vulval SCC
Vulval SCC and associated precursor lesions develop through two pathways (Figure 1) and have different clinical features depending on the pathway (Table 2).2 Traditionally most vulval SCC arose from the HPV-independent pathway, but the epidemiology has now changed in New Zealand and the proportions of vulval SCC from both pathways are almost equal.4
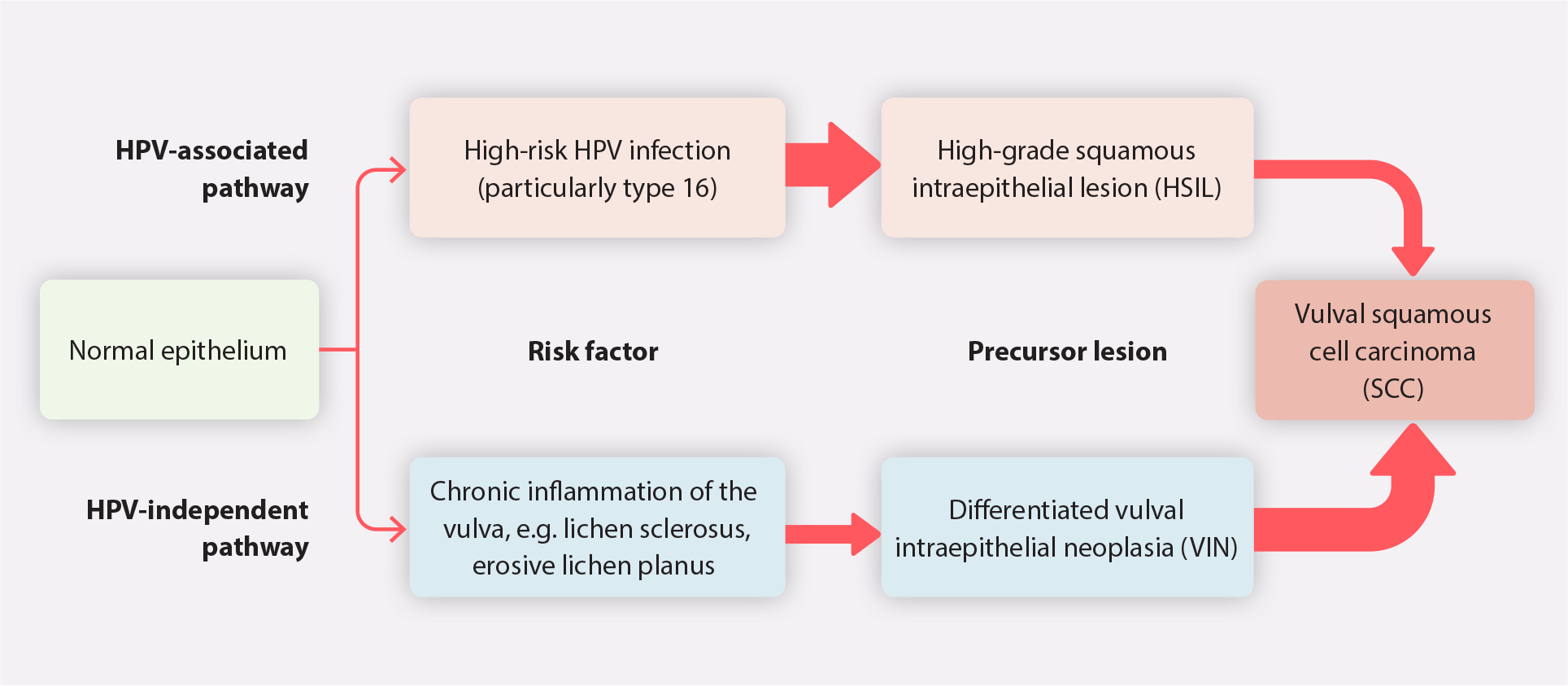
Figure 1. An overview of the HPV-independent and HPV-associated pathways for the development of vulval squamous cell carcinoma.2, 5, 9, 16 N.B. The larger arrows denote a higher likelihood of progression. HPV = human papillomavirus.
The HPV-independent pathway is usually associated with lichen sclerosus (or other chronic inflammation)*, differentiated VIN† and older age.2, 5, 9 In contrast, the HPV-associated pathway is associated with high-risk HPV infection, high-grade squamous intraepithelial lesions (HSIL) and a younger age at diagnosis (peak prevalence at ages 40 – 44 and > 50 years).2, 9, 17 The prevalence of HPV-associated vulval SCC is increasing in females aged > 50 years in New Zealand.4
*Other factors may also contribute to the development of differentiated VIN, e.g. mutations in tumour suppressor gene, p53 2, 5
†Differentiated VIN is considered the main precursor lesion associated with the HPV-independent pathway; other precursor lesions may include differentiated exophytic vulvar intraepithelial lesions (DEVIL) and vulvar acanthosis with alternative differentiation (VAAD)16
The majority of vulval cancer precursor lesions (approximately 95%)11 are HSIL (previously referred to as usual-type VIN, VIN 2/3 or Bowen disease of the vulva), but these are associated with a low risk and rate of progression to invasive vulval cancer.5, 15, 16 Data vary across the literature; some studies report that < 5% of HSIL progress to invasive vulval cancer, while others report a risk of progression of approximately 10% (within ten years in females with treated vulval HSIL; the risk is likely to be higher in females with untreated vulval HSIL).5, 15, 16 For information on the treatment and follow-up of a patient with HSIL, see: “Vulval HSIL”.
In comparison, differentiated VIN, although less common than HSIL has a high risk and rapid rate of progression to invasive vulval cancer.** 16–19 Estimates vary but approximately 50% of females with differentiated VIN develop vulval SCC within ten years with a median progression time of one to two years.15 A small local study found that the median progression time between biopsy showing differentiated VIN and invasive cancer was 43.5 months.20 For information on the treatment and follow-up of a patient with differentiated VIN, see: “Differentiated VIN”.
**The low prevalence of differentiated VIN may be due to the rapid rate of progression to invasive vulval cancer and underdiagnosis of the lesion5
Summary of vulval squamous cell carcinoma
- Most vulval cancers are squamous cell carcinoma (SCC)
- Vulval SCC usually develop via two pathways: HPV-independent (typically related to lichen sclerosus or other chronic inflammation of the vulva and associated with differentiated VIN) and HPV-associated (related to HPV infection and associated with HSIL)
- The majority of precursor lesions are HSIL from the HPV-associated pathway
- However, the precursor lesions associated with the HPV-independent pathway (differentiated VIN) are more likely to progress to invasive squamous cell vulval cancer
- Therefore the HPV-independent and HPV-associated pathways are responsible for almost equal numbers of squamous cell vulval cancers
Rare types of vulval cancer
The remaining 10% of vulval cancers predominantly include (see Table 2 for details on how to visually identify these types):
- Primary vulval melanoma. Vulval melanoma is not related to exposure to sunlight or UV radiation; it can occur at any age but is most common among Europeans aged 40 – 60 years.21 Patients with vulval melanoma are typically diagnosed at a later stage and at an older age than those with other melanomas.5
- Bartholin gland carcinoma. A mass in the location of a Bartholin’s gland is more likely to be malignant in patients who are aged ≥ 40 years compared to patients who are younger, particularly if it is fixed, firm or irregularly shaped.22 Therefore, conservative management of Bartholin cysts with Word catheters (a flexible tube with a small balloon that is inserted into a cyst or abscess for drainage) in older females is not recommended.
- Basal cell carcinoma (BCC). Usually diagnosed in females who are post-menopausal at an average age of 70 years.9, 21 Unlike BCC at other sites, vulval BCC is not associated with exposure to sunlight or UV radiation, although risk may be increased by immunosuppression, chronic irritation, pelvic radiation or trauma.21 The risk of metastases or recurrence is low and long-term follow-up is not usually required.9
- Paget disease of the vulva. Paget disease of the vulva typically affects females who are post-menopausal aged in their 60s of European ethnicity.2, 5 It is usually a pre-cancerous intraepithelial lesion (adenocarcinoma in situ), but can progress to invasive adenocarcinoma.2, 5, 16 Invasive Paget disease of the vulva accounts for 1 – 2% of vulval cancers.2, 5 In fewer than 10% of cases, Paget disease of the vulva can be associated with other cancers, and further investigations, e.g. mammogram, urine cytology/cystoscopy, are often recommended.
The diagnosis of vulval cancer involves the recognition of suspicious vulvovaginal symptoms from a focused patient history, followed by investigation with a pelvic examination to detect any abnormal changes to vulval appearance.3, 8 Referral to a gynaecologist is required for patients with suspicion for vulval cancer for consideration of a vulvoscopy or biopsy (diagnosis is made via biopsy).
Most people with vulval cancer are symptomatic
While a small number of people with vulval cancer (or precursor lesions) are asymptomatic, most experience some degree of symptoms such as vulval pruritus, pain, irritation, bleeding or discharge.3, 5, 16 A vulval lesion (i.e. mass or ulcer) is generally present.5, 9, 16 A study in the United Kingdom found that the risk of vulval cancer was 12.8% in patients with a suspicious vulval lesion and this risk was higher if the lesion was painful or bleeding.2 In some cases, the patient or their partner may have noticed a lesion themselves, or it may have been detected by a clinician during a pelvic examination for another reason, e.g. cervical screening or follow-up of patients with lichen sclerosus.3
Take a history and perform a vulval examination for patients with suspicion of vulval cancer
Begin by taking a focused history with particular attention given to vulvovaginal symptoms and any risk factors, e.g. lichen sclerosus. Perform a pelvic examination, including speculum or bimanual examination, as indicated.3, 8, 9 Palpate the groin to assess for any enlarged lymph nodes.9 Offer cervical screening to patients who are due. Swabs for sexually transmitted infections, e.g. genital herpes, are not usually indicated if a tumour is present, but may be useful if there is ulceration, bleeding or discharge, to exclude other potential causes.
Examining the vulval region
When examining the vulval region, pay close attention to the labia majora as this is the most common site of vulval cancer.8, 9 Changes to vulval appearance due to precursor lesions or vulval cancer are highly variable (see Table 2 for specific features). In general, look for:2, 3, 5
- Changes to the surface of the skin, i.e. irregular epithelial surfaces
- Swelling
- Changes in colour, e.g. red, white or other pigmented areas
- A visible lesion, e.g. flat, raised, lump (or apparent genital warts in a post-menopausal patient)
- Ulceration or bleeding
Table 2. Typical features of vulval cancer types and precursor lesions.
HSIL/HPV-associated vulval SCC |
Lesions have a variable appearance, but may be multifocal with red, white or other pigmented areas18 |
Differentiated VIN/HPV-independent vulval SCC |
Unifocal, unicentric, rough, warty plaques or papular lesions with poorly defined edges.16, 18 Plaques are usually pink or grey-white and hyperkeratotic.16 Ulceration may be present.18 Can be difficult to distinguish from underlying lichen sclerosus.16, 18 |
Vulval melanoma |
Asymmetric macule, papule or nodule with irregular margins, with or without ulceration or pigmentation (i.e. amelanotic melanoma).2, 5 Often on the labia majora, labia minora or clitoris.5 The ABCDEFG checklist may help to differentiate vulval melanoma from benign naevi and melanosis of the vulva.
For further information on melanoma, including use of dermatoscopy, see: bpac.org.nz/2021/melanoma-detection.aspx |
Bartholin gland carcinoma |
Painful mass in the area of a Bartholin’s gland that is firm, fixed or nodular on palpation.2 Age > 40 years should raise suspicion for malignancy.2 A regularly shaped mass that is soft, mobile and often tender to touch is more likely to be a Bartholin’s gland cyst or abscess than carcinoma.22 |
Vulval BCC |
Discrete solitary nodule, papule or plaque, or raised, rolled-edge ulcer, usually on the labia majora2, 9 |
Paget disease of the vulva
(adenocarcinoma in situ and adenocarcinoma) |
Painful and erythematous (or pink) rash or eczematous lesion/plaque, typically asymmetric or unilateral on the labia majora.2, 5 Can be difficult to distinguish from other inflammatory dermatoses. |
 Refer patients with a lesion (or lesions) suspected to be VIN or vulval cancer to a gynaecologist for further assessment which is likely to include vulvoscopy and/or biopsy.3 A fungating mass or palpable inguinal nodes indicate more advanced cancer and should prompt urgent referral.2
Refer patients with a lesion (or lesions) suspected to be VIN or vulval cancer to a gynaecologist for further assessment which is likely to include vulvoscopy and/or biopsy.3 A fungating mass or palpable inguinal nodes indicate more advanced cancer and should prompt urgent referral.2
 Patients with generalised vulval irritation but no visible lesion on examination are unlikely to have vulval cancer.2 Consider other causes of the patient’s symptoms, including candidiasis, an inflammatory dermatosis, e.g. lichen sclerosus or planus, or a sexually transmitted infection, e.g. genital herpes.18
Patients with generalised vulval irritation but no visible lesion on examination are unlikely to have vulval cancer.2 Consider other causes of the patient’s symptoms, including candidiasis, an inflammatory dermatosis, e.g. lichen sclerosus or planus, or a sexually transmitted infection, e.g. genital herpes.18
The biopsy results may indicate the presence of a vulval condition such as lichen sclerosus or Paget disease of the vulva, or it may show a precursor or malignant lesion. If the biopsy results indicate vulval cancer, the patient will be managed in a Gynaecological Oncology centre in Auckland, Wellington or Christchurch. Patients whose biopsy results show pre-cancerous lesions or inflammatory conditions of the vulva (see below) may require treatment to reduce the risk of progression to invasive vulval cancer and ongoing monitoring due to the risk of recurrence.18 Consider setting a recall for periodic follow-up of these patients.
Lichen sclerosus
Effective control of lichen sclerosus with topical corticosteroids reduces the risk of progression to differentiated VIN and invasive vulval cancer.23, 24 Patients should be reviewed at least annually, or more often if they notice changes to their symptoms or to the feel or appearance of their lesion(s).16, 25 Referral for an additional biopsy should be considered in patients with lichen sclerosus who do not respond to treatment.5, 25 Long-term or indefinite follow-up for a patient with lichen sclerosus is required; those with stable well controlled lichen sclerosus may be followed up in primary care (with periodic specialist review).25, 26
For further information on lichen sclerosus, see: bpac.org.nz/bpj/2014/september/vulvovaginal.aspx
Paget disease of the vulva
Consider investigating for co-existing malignancies, as a diagnosis of Paget disease of the vulva may represent spread of an adenocarcinoma from another site, e.g. rectum, bladder, urethra, cervix.2
There are multiple treatment options available for patients diagnosed with Paget disease of the vulva with the overall aim being to reduce the risk of progression to invasive adenocarcinoma. Surgery is usually the standard treatment, but imiquimod cream (unapproved indication) is an effective non-surgical option for some patients.2, 16 As the rate of recurrence after treatment is high (up to 70%), long-term follow-up is recommended.2
For further information on Paget disease of the vulva, see: dermnetnz.org/topics/extramammary-paget-disease
Low-grade squamous intraepithelial lesion
Low-grade squamous intraepithelial lesions (LSIL) indicate infection with HPV (in most cases with low risk types, 6 or 11), and do not require treatment.16 The infection is usually transient and self-resolves within one to two years.27 LSIL are classified as low risk but progression to HSIL is possible (uncommon); consider setting a recall for periodic follow-up until the lesion resolves.27
High-grade VIN
Surgical resection is often the first-line treatment for patients with differentiated VIN or vulval HSIL as these lesions (especially differentiated VIN) are associated with an increased risk of invasive vulval cancer.16, 18 Recurrence is common after treatment for high-grade VIN, so patients may benefit from increased surveillance.16, 18
Differentiated VIN
Differentiated VIN need to be excised. After excision, treatment of any underlying lichen sclerosus with ultrapotent topical corticosteroids is recommended to reduce the risk of recurrence and subsequent progression to vulval SCC.16, 19 European guidelines recommend ongoing follow-up at least every six months, depending on the severity of any associated lichen sclerosus.18
Vulval HSIL
First-line treatment of vulval HSIL may include surgical excision or the topical application of imiquimod cream (unapproved indication).2, 16 Exclusion of malignancy by mapping biopsies may be performed prior to treatment.16 Recurrence of vulval HSIL after treatment is high at 20 – 40%.17
Vulval HSIL frequently co-exists with other HPV-associated cancers or pre-cancerous lesions. A history of cervical cancer or abnormal cervical cytology is present in 50% of females diagnosed with vulval HSIL and up to half of those diagnosed with vulval HSIL develop another HPV-associated genital cancer or pre-cancerous lesion, e.g. cervical, vaginal or anal.27 As the incidence of anal cancer is higher in people with a history of pre-cancerous vulval lesions or vulval cancer, consider investigations for anal cancer, e.g. digital anorectal examination, anal cytology, as indicated.2, 16
For patients with treated cervical HSIL, New Zealand Cervical Screening Guidelines recommend HPV and cytology testing (test of cure) before returning to their regular cervical screening interval (if both tests are negative/normal at 6 and 18 months post-treatment);28 there are no specific cervical screening recommendations for people treated for vulval HSIL. International guidelines recommend that all patients with a history of vulval HSIL have annual cervical screening (it is unclear whether this will change when HPV testing is introduced).18 In practice, decisions around cervical screening for those with a history of vulval HSIL will be made on a case-by-case basis in conjunction with a gynaecologist.
For information on the follow-up and surveillance of a patient after curative-intent treatment for vulval cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
 There is a B-QuiCK summary available for this topic
There is a B-QuiCK summary available for this topic





