Published: 9 February 2023
Key practice points:
- There are three main types of ovarian cancer: epithelial ovarian cancer accounts for more than 90% of all diagnoses, followed by sex cord-stromal tumours and malignant germ cell tumours
- The lifetime number of ovulatory cycles is one of the most significant risk factors for developing ovarian cancer. People may also have a genetic predisposition, e.g. family history of breast or ovarian cancer, BRCA mutation, Lynch syndrome (see main text for a full list of risk factors). Ovarian cancer incidence is higher in Pacific peoples compared with other ethnic groups.
- Recognising and investigating suspicious symptoms, which are often non-specific in isolation, is essential to the early detection of ovarian cancer
- Symptoms may include abdominal or pelvic pain, abdominal bloating or distention, loss of appetite, early satiety and increased urinary frequency or urgency
- There should be a higher suspicion of ovarian cancer for a patient who is post-menopausal and/or if symptoms are new, severe, unusual, recurrent or persistent, e.g. occurring at least 12 days per month
- For all patients with suspected ovarian cancer, perform a physical examination, including bimanual examination feeling for any abdominal or adnexal mass or ascites, request a serum CA 125 test and refer for a pelvic ultrasound (if indicated, see below)
- Refer patients with abnormal examination findings or an elevated CA 125 level for a pelvic ultrasound. Consider other potential diagnoses for patients who do not initially meet the criteria for a pelvic ultrasound. If symptoms persist or worsen, reassess for ovarian cancer.
- Some females with a personal or family history of cancer (e.g. breast, ovarian, endometrial, colorectal) or a known cancer gene mutation in a blood relative (e.g. BRCA1/2) may be eligible for, and benefit from, genetic testing. Bilateral salpingo-oophorectomy when childbearing is complete reduces the risk of ovarian cancer in females with hereditary cancer syndromes.
N.B. The term “female” is used in this article to describe the biological sex of the patient population at risk for ovarian cancer. However, we acknowledge that this may not reflect the identity of all patients, which will include transgender boys or men, intersex and non-binary individuals.
For information on the follow-up and surveillance of a patient after curative-intent treatment for ovarian cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
Ovarian cancer is the second most common gynaecological cancer in New Zealand with an average of 371 new diagnoses (9.9 per 100,000 females; from 2015 – 2020) each year;* more than cervical cancer but fewer than endometrial cancer.1 In 2020, it was the seventh most common cancer in females.1 The risk of ovarian cancer increases with age and it primarily affects females who are post-menopausal. This is reflected in New Zealand data where incidence first peaks between the ages of 45 – 64 years, and again in those aged > 75 years.2
There is no effective screening test for ovarian cancer, so diagnosis relies on the prompt recognition and investigation of suspicious symptoms (see: “Diagnosing ovarian cancer”).3 ,4 While ovarian cancer was historically considered to be a silent disease in its early stages, evidence suggests that 90 – 95% of people are symptomatic.3, 5 However, because symptoms are often non-specific, it is estimated that only one in 400 – 600 females who report symptoms has an ovarian malignancy.3, 6 Females with ovarian cancer in New Zealand reportedly visit their general practitioner an average of three to five times before having diagnostic tests for ovarian cancer.7
*There are differences in published data. For consistency across the gynaecological cancer series, incidence and mortality data has primarily been obtained from the publication – Cancer: Historical summary 1948 – 2020.
Ovarian cancer incidence and mortality remain steady over time
The overall incidence and mortality from ovarian cancer in New Zealand have remained relatively steady over time (Figure 1).1 As many people are diagnosed with ovarian cancer at an advanced stage, mortality rates are high (240 deaths on average each year from 2015 – 2018*; 5.7 per 100,000 females).8 Ovarian cancer has the highest mortality of all gynaecological cancers in New Zealand, causing more deaths than the others combined.1
*Mortality data are available for 2019, but are preliminary so have not been included. Mortality data for 2020 are not yet available.
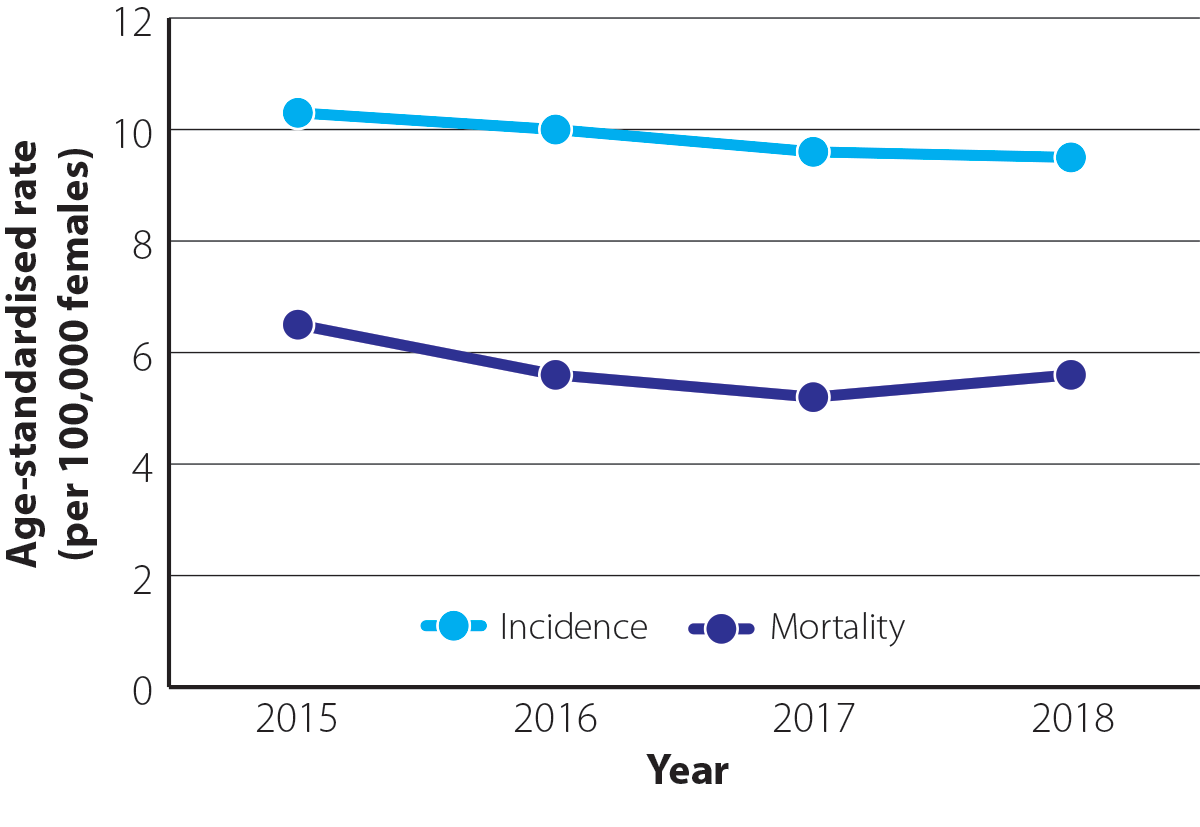
Figure 1. Age-standardised incidence and mortality rates (per 100,000 females) for ovarian cancer between 2015 and 2018 in New Zealand.1N.B. Incidence data are available up to 2020 but have not been included in this graph for comparative purposes. Mortality data are available for 2019, but are preliminary so have not been included. Mortality data for 2020 are not yet available.
Ovarian cancer is more common in Pacific peoples compared with other ethnic groups (Figure 2). In 2019 (most recent ethnicity data available), the age-standardised incidence rate for Pacific peoples was 19.5 per 100,000 females which was 2.0 times greater than the rate in Māori (9.6 per 100,000 females) and 2.2 times greater than in European/Other ethnic groups (8.9 per 100,000 females).9
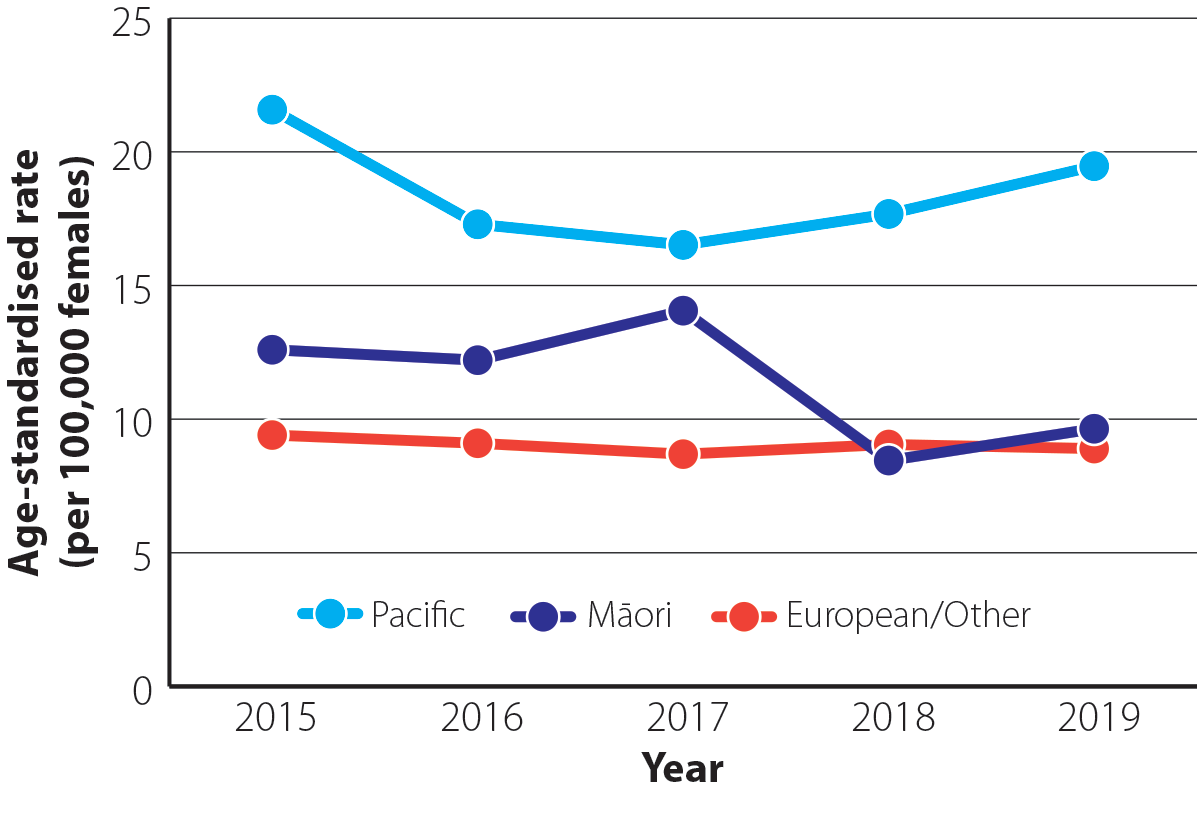
Figure 2. Age-standardised incidence rate (per 100,000 females) for ovarian cancer by ethnicity between 2015 and 2019 in New Zealand.9 N.B. Incidence data by ethnicity are not yet available for 2020.
Ovarian cancer has a high mortality rate, but five-year survival rates are improving
The five-year survival rate for people with ovarian cancer in New Zealand improved from 32% in the 1990s to 36% between 2010 and 2014.10 However, this remains lower than five-year survival in Australia (43%), Canada (40%) and the United Kingdom (37%) between 2010 and 2014.10 This variation may be partly explained by differences in the threshold for investigating suspicious symptoms, criteria for referral and access to oncology treatments.
Survival rates progressively decrease according to the stage of ovarian cancer and degree of spread (Table 1).11 Reducing the time from presentation to referral should therefore be a key focus in primary care to help improve survival in line with other countries.
Table 1. Three-year survival rate for people with ovarian cancer in New Zealand by stage between 2010 – 2014.12, 13
| Degree of spread |
FIGO*stage |
Description |
Three-year survival rate |
| Localised |
Stage I |
Confined to one or both ovaries |
99.8% |
| Regional |
Stage II/III |
Spread within the pelvis (uterus, fallopian tubes, bladder or bowel) or to the peritoneum or local lymph nodes |
69.8% |
| Distant |
Stage IV |
Spread to distant organs |
31.6% |
*International Federation of Gynecology and Obstetrics
Epithelial ovarian cancer is the most common type of ovarian cancer
Ovarian cancer is classified into three main types:14
- Epithelial ovarian cancer – represents more than 90% of ovarian cancer diagnoses. This type of ovarian cancer is further classified into multiple subtypes (Figure 3); approximately 70% of epithelial ovarian cancers are classified as high-grade serous carcinoma.
- Sex cord-stromal tumours, e.g. granulosa cell tumours – median age at diagnosis is 58 years and only 12% occur in females aged under 40 years15
- Malignant germ cell tumours, e.g. teratomas – most common in females aged in their 20’s and two-thirds of diagnoses occur before age 40 years8, 15
High-grade serous carcinomas, the most common type of ovarian cancer, usually originate in the fimbria portion of the fallopian tubes.16 These tumours grow quickly and spread early, therefore they are usually diagnosed at a late stage where prognosis is poor.17 Early diagnosis is more likely in females with other subtypes of epithelial ovarian cancer where growth is slower.17 Prognosis is generally better for people with non-epithelial ovarian cancer than those with epithelial ovarian cancer, however, this is dependent on the stage of cancer and age at diagnosis.15
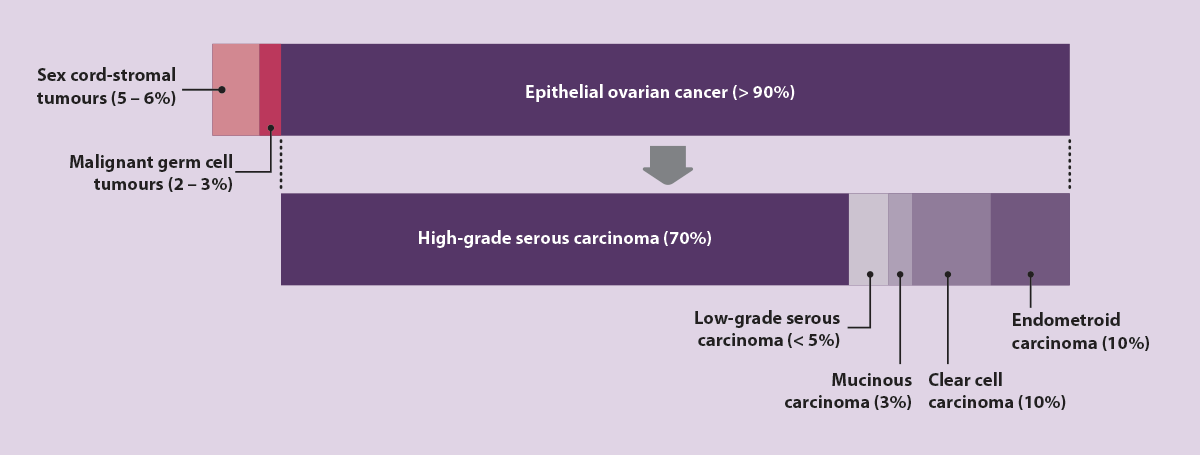
Figure 3. Types of ovarian cancer, including subtypes of epithelial ovarian cancer, by frequency of diagnosis.14
Several factors influence the risk of developing ovarian cancer and certain exposures can have different effects on the various subtypes.18 Significant risk factors, and potential associations (Table 2), are listed below.
Ovulation
One of the most significant risk factors for epithelial ovarian cancer is the lifetime number of ovulatory cycles.14 Factors that increase the number of ovulatory cycles therefore increase the risk of ovarian cancer, e.g. early menarche, late menopause, nulliparity.14 Conversely, factors that interrupt ovulation can reduce ovarian cancer risk, e.g. oral contraceptive use (see below), pregnancy, breastfeeding, early onset menopause.14, 19 N.B. Other forms of contraception that disrupt ovulation may also reduce the risk of ovarian cancer, e.g. some studies link use of an intra-uterine device with a lower risk of ovarian cancer, however, further studies are required to provide definitive evidence.14
Repeated exposure to inflammation occurring in the distal fallopian tubules after ovulation may contribute to the increased risk of ovarian cancer over time, but the exact mechanism is unknown.20 An alternative theory is that a greater number of ovulatory cycles is associated with an increased likelihood of spontaneous mutations from more cellular divisions, and therefore, an increased risk of malignancy.18
Oral contraceptive use, ovarian and breast cancer
The use of the oral contraceptive pill has been shown to reduce the risk of ovarian cancer both in the general population and in those with a genetic predisposition, e.g. BRCA mutation, by approximately 50%.21 There is greater protection with increasing duration of use.21 Protective effects persist after cessation, but reduce over time.21
However, there is conflicting evidence about whether use of the oral contraceptive pill is associated with an increased risk of breast cancer.21 Most studies report little to no increased risk of breast cancer and any effect appears to be temporary and limited to current or recent (within five years) use.21 For most people, without other risk factors, the protective effect against ovarian cancer is likely to be greater than the potential increased risk of breast cancer, but this should be part of a risk-benefit discussion if considering this method of contraception. Combined oral contraceptive pills should not be used in patients with current breast cancer and use is strongly cautioned against in those with a history of breast cancer or who are known carriers of genetic mutations associated with breast cancer, e.g. BRCA1 or BRCA2.22
Genetics
Females with a family history of breast or epithelial ovarian cancer are at a significantly increased risk of developing ovarian cancer due to an inherited mutation.19 Mutations in BRCA1 and BRCA2 genes are the predominant genetic cause, with a small number of cases due to Lynch syndrome.19 Hereditary cancer syndromes are linked to 10 – 12% of ovarian cancer diagnoses;8 some sources report an even higher association.18 For further information, see: “Hereditary cancer syndromes”.
Table 2. Additional factors that are potentially associated with an increased risk of ovarian cancer.
 N.B. Cumulative number of ovulatory cycles and genetics are the main risk factors (see text).
N.B. Cumulative number of ovulatory cycles and genetics are the main risk factors (see text).
| Lifestyle factors |
Benign gynaecological conditions |
| Obesity. There is a potential association between obesity and ovarian cancer (possibly through the production of oestrogen in adipose tissue), however, evidence is limited and inconsistent.23 The influence of physical activity as a protective measure for ovarian cancer remains inconclusive.14 Exercise should still be encouraged due to the additional benefits on weight control and overall health. |
Endometriosis is associated with an increased risk of epithelial ovarian cancers.14, 24 It is unclear whether this association is causal or the result of shared risk factors or signalling pathways.14 |
| Polycystic ovary syndrome may increase the risk of ovarian cancer;14 data are limited and studies are often confounded by infertility and obesity.25 |
| Cigarette smoking is associated with an increased risk of the mucinous subtype of epithelial ovarian cancer.14 |
Pelvic inflammatory disease has been associated with an increased risk of ovarian cancer in some studies.26 The chronic inflammation associated with pelvic inflammatory disease has been proposed as a cause, however, evidence is limited.26 |
| Alcohol and Diet. There is inconsistent evidence as to whether alcohol consumption or specific dietary factors, e.g. consumption of saturated fats, high protein intake, are associated with an increased risk of ovarian cancer.14 |
| Other factors |
| Long term use of menopausal hormone therapy (i.e. > 5 years) is associated with a small increased risk of ovarian cancer.14 Risk reduces after cessation. The risk of ovarian cancer may be higher in people taking oestrogen-only menopausal hormone therapy as increased exposure to oestrogen unopposed by progesterone can stimulate proliferation of epithelial ovarian cells, but data are inconsistent.14 |
| Assisted reproduction. There is little evidence of an association between the use of medicines that stimulate ovulation, e.g. clomifene citrate or gonadotropin therapy, and an increased risk of ovarian cancer.27 Nulliparity is often a confounding factor.27 There is low-certainty evidence of an association between in vitro fertilisation and an increased risk of borderline ovarian tumours.27 |
| Talcum powder. There may be an association between the regular application of talcum powder in the genital area and an increased risk of ovarian cancer, although evidence is unclear.14 |
Risk factors for non-epithelial ovarian cancer
Risk factors for sex cord-stromal tumours and malignant germ cell tumours are poorly understood due to the rarity of these. There is some evidence, however, that multiple pregnancies and use of the oral contraceptive pill may be protective against sex cord-stromal tumours.15
The diagnostic pathway for ovarian cancer involves the recognition of symptom patterns from a focused patient history, followed by investigation with a physical examination, including bimanual pelvic examination, serum CA 125 test and a pelvic ultrasound, if indicated (Figure 4).3 There is no evidence to support the use of investigations to detect ovarian cancer in people who are asymptomatic, including high-risk groups (see: “Hereditary cancer syndromes”).3, 4

*Avoid measuring CA 125 during menstruation if possible. Also measure human chorionic gonadotrophin and alpha fetoprotein if a patient is pre-menopausal.
†Should include transvaginal ultrasound
Figure 4. A suggested approach to the diagnosis of ovarian cancer.3, 11
Symptoms of ovarian cancer tend to be non-specific and are commonly related to another cause
Most people (70%) with early-stage ovarian cancer have been reported to present with one or more non-specific symptoms; abdominal or pelvic pain are usually the most common (see full list below).5 In isolation, symptoms have a low positive predictive value for ovarian cancer.6 Symptoms are more likely to be associated with ovarian cancer if they are in a post-menopausal female and/or if they are new, severe, unusual, recurrent or persistent, e.g. occurring 12 or more days per month.3, 4
Symptoms of ovarian cancer may include:3–5
- Abdominal bloating or distension
- Abdominal or pelvic pain
- Early satiety or loss of appetite
- Increased urinary frequency or urgency
- Changes in bowel habit*
- Fatigue
- Unexplained weight loss
- Post-menopausal bleeding
*New symptoms suggestive of irritable bowel syndrome (IBS) in females aged over 50 years are unusual; consider the possibility of ovarian cancer in these patients4, 11
A daily symptom diary can help to quantify which symptoms the patient is experiencing and how often.17 A printable template for recording symptoms is available from: www.ovariancancer.net.au/page/134/signs-and-symptoms
Consider how education about the key symptoms of ovarian cancer might be incorporated during relevant discussions to minimise delayed presentation and to improve the likelihood of early detection.
Perform a physical examination and request laboratory tests for patients with symptoms of ovarian cancer
Palpate and percuss the abdomen to check for a mass or signs of ascites.11 Also perform a bimanual examination to feel for adnexal masses, and check for lymphadenopathy.
Request a serum CA 125 test for all patients with symptoms suggestive of ovarian cancer (see: “The changing role of cancer antigen 125 in primary care”).3, 11 N.B. Menstruation can raise CA 125 levels; avoid testing during this time.3 In pre-menopausal females, also measure human chorionic gonadotrophin and alpha fetoprotein, which may be elevated in those with a malignant germ cell tumour.11
Additional laboratory tests may be requested (as indicated) to assess other aspects of the patient’s health and to identify other potential causes of the symptoms.
Tests may include:
- Full blood count
- Liver function tests
- CRP
- Calcium
- Creatinine and urea
- Electrolytes
The changing role of cancer antigen 125 in primary care
CA 125 is cell-surface glycoprotein that is primarily measured to aid in the diagnosis and management of ovarian cancer. CA 125 is raised in more than 80% of people with advanced epithelial ovarian cancer, but only in 50% of those with early-stage disease.28 It is less commonly raised in people with non-epithelial ovarian cancers. Higher levels of CA 125 should increase suspicion of malignancy; a level of > 200 U/mL is suggestive of malignancy or > 65 U/mL with an ovarian mass, but there is no level that is exclusively associated with cancer.28
Some non-malignant conditions are also associated with raised CA 125 levels. These may include conditions involving or inflaming the peritoneum, pericardium or pleura, e.g. endometriosis, pelvic inflammatory disease, ascites, recent abdominal surgery, pleuritis, pericarditis or congestive heart failure with pleural effusion.28 Menstruation and first-trimester pregnancy can also cause up to three-fold elevations in CA 125.28
CA 125 is not appropriate as a screening test for ovarian cancer due to its low sensitivity and specificity.28 Instead it is used to determine which females presenting with symptoms suggestive of ovarian cancer should be prioritised for further assessment. Occasionally after a diagnosis of ovarian cancer, CA 125 is used to monitor response to treatment.28
Refer patients with abnormal examination findings for a pelvic ultrasound
If there are suspicious findings on physical examination, refer the patient for an urgent pelvic ultrasound,* irrespective of CA 125 level.11 The wait time for an ultrasound varies by region and by the degree to which cancer is suspected. In some situations it may be appropriate to discuss options for a privately funded ultrasound with the patient. If the results from the pelvic ultrasound are abnormal, refer the patient to a gynaecologist for further assessment.
*Pelvic ultrasound for patients with suspected ovarian cancer usually includes a transvaginal ultrasound. It is recommended that this is discussed with patients prior to the referral so they know what to expect and because some patients may decline.
Manage patients with normal examination findings according to CA 125 level and menopausal status
Pre-menopausal:
- CA 125 level < 35 U/mL – consider differential diagnoses. Advise patients to return for re-evaluation if their symptoms worsen or persist for longer than three months. Recommend that they continue to keep a symptom diary which may help to quantify symptoms.17
- CA 125 level 35 – 200 U/mL – repeat serum CA 125 in six weeks:
- If the CA 125 level has increased or remains elevated, refer the patient for a pelvic ultrasound and then discuss with or refer to a gynaecologist
- If the CA 125 level has decreased, reassure the patient that it is unlikely that they have ovarian cancer; no further investigation is required unless symptoms worsen
- CA 125 level > 200 U/mL – refer the patient for a pelvic ultrasound. Once the ultrasound results are obtained, refer the patient to a gynaecologist.
Post-menopausal:
- CA 125 level < 35 U/mL – consider differential diagnoses. Advise patients to return for re-evaluation if their symptoms worsen or persist for longer than three months. Recommend that they continue to keep a symptom diary which may help to quantify symptoms.17
- CA 125 level > 35 U/mL – refer the patient for a pelvic ultrasound. Once the ultrasound results are obtained, refer the patient to a gynaecologist.
Risk of malignancy index may be calculated prior to secondary care referral
Some HealthPathways recommend calculating the risk of malignancy index (RMI) and including this in addition to the CA 125 result when requesting gynaecology referral; mark the referral as urgent if the RMI is high. The RMI uses menopausal status, CA 125 level and ultrasound results to estimate the likelihood that an identified mass is ovarian cancer. The RMI is recommended to be calculated by some international guidelines to guide decisions around referral to a gynaecological oncologist.11 However, there is a lack of consensus on threshold levels for referrals and regardless of the RMI, patients with symptoms, an abnormal ultrasound or raised CA 125 level should be referred to secondary care.
For information on how to calculate the RMI, see Figure 4. A RMI calculator is also available from: www.mdcalc.com/calc/10103/risk-malignancy-index-rmi-ovarian-cancer
Reassess the patient if symptoms persist
For patients who were not initially referred to secondary care, but have unexplained symptoms that are persistent or worsening, reconsider differential diagnoses, including ovarian cancer as a cause.11 It may be appropriate to repeat serum CA 125 testing, reconsider referral for a pelvic ultrasound and/or discuss with or refer the patient to a gynaecologist.3
Mutations in BRCA1 and BRCA2 genes are associated with an increased risk of breast, ovarian and (less commonly) pancreatic cancer (Table 3).29 It is estimated that 1 in 300 – 800 females has a BRCA mutation.8 Approximately 10% of people diagnosed with ovarian cancer have a BRCA mutation.8
Lynch syndrome is an inherited condition that results from a germline mutation in one of the four DNA mismatch repair genes.30 It is associated with increased risk of colorectal, endometrial and ovarian cancers, among others (Table 3).30 The estimated prevalence of Lynch syndrome is 1 in 279 people (males and females).30 It is implicated in 2 – 3% of ovarian cancer diagnoses.8
BRCA genes and the DNA mismatch repair genes associated with Lynch syndrome are inherited in an autosomal dominant pattern, meaning that children of a carrier have a 50% chance of inheriting the mutation.30
Table 3. Cumulative risk of cancer in females with a hereditary cancer syndrome.29–31
| Mutation |
Risk of ovarian cancer |
Other associated cancers |
BRCA1 |
44% to age 80 years |
Breast
Pancreatic |
(72% risk to age 80 years)
(1% risk to age 70 years) |
BRCA2 |
17% to age 80 years |
Breast
Pancreatic |
(69% risk to age 80 years)
(2% risk to age 70 years) |
Lynch syndrome |
6% to age 70 years |
Colorectal
Endometrial
Urothelial
Breast
Small bowel
Gastric |
(41% risk to age 70 years)
(36% risk to age 70 years)
(13% risk to age 70 years)
(12% risk to age 70 years )
(12% risk to age 70 years)
(3% risk to age 70 years) |
There is insufficient evidence to recommend testing for ovarian cancer (using serum CA 125 and pelvic ultrasound) in people who are asymptomatic with a hereditary cancer syndrome.3
A pragmatic approach in New Zealand may be to consider referral to the Genetic Health Service (see: “Referral for genetic testing”) and to provide education about potential symptoms of ovarian cancer alongside encouragement to present to primary care early if these symptoms are experienced.
Risk-reducing surgery for people with a hereditary cancer syndrome
A bilateral salpingo-oophorectomy is recommended for females with a BRCA mutation after childbearing is complete (or not desired), ideally between ages of 35 – 40 years.3 This reduces the risk of ovarian cancer by at least 90%.14 In BRCA mutation carriers, pre-menopausal bilateral salpingo-oophorectomy also reduces breast cancer risk by more than 50%.3 Removal of the fallopian tubes for people with a BRCA mutation is also important as these are the site of origin for some ovarian cancers.3, 16 Some studies have reported a 30 – 40% decrease in ovarian cancer risk after a tubal ligation or hysterectomy.14
Hysterectomy is not routinely recommended for people with a BRCA mutation, but some may opt to have their uterus removed if they are planning to take oestrogen-only menopausal hormone therapy or prophylactic tamoxifen, which are otherwise associated with an increased risk of endometrial cancer.32 People with Lynch syndrome may opt for a hysterectomy with bilateral salpingo-oophorectomy to reduce the risk of both endometrial and ovarian cancer.33
Referral for genetic testing

Genetic testing can improve the management of people with mutations associated with an increased risk of cancer,
particularly through risk-reducing measures such as prophylactic surgery.3, 34
Some patients can be referred for hereditary cancer assessment if they have a blood relative with a known cancer gene mutation, e.g. BRCA1/2, or a personal or family history of cancer, in particular breast, ovarian or a Lynch syndrome-associated cancer, e.g. endometrial or colorectal.3, 34 Check local HealthPathways to assess eligibility criteria for referral to Genetic Health Service New Zealand.
Genetic testing should also be considered in patients after being diagnosed with breast or ovarian cancer, as the results may modify choice of treatment.34 This provides another pathway to identify a hereditary cancer gene in a family.
Genetic Health Service New Zealand uses referral guidelines determined by eviQ; for further information, see: www.eviq.org.au/cancer-genetics/referral-guidelines/1905-ovarian-cancer-referring-to-genetics
Further information on Genetic Health Service New Zealand is available from: https://www.tewhatuora.govt.nz/health-services-and-programmes/genetic-health-service-nz/
Risks of genetic testing
Genetic testing is usually offered alongside genetic counselling, where the risks associated with testing will be discussed.
Testing for hereditary cancer may be distressing, particularly in people who find out that they have an increased risk of cancer. Prior to referring the patient for genetic testing, explain what is being tested, the implications for personal and family risk and how the results might change their future medical care. Encourage patients to discuss the test with family members, especially those who may also be at increased risk.
An inconclusive result is possible when a novel mutation to a known gene is found. As research into variants is ongoing, some people will learn the significance of their mutation many years after their test.
There are potential financial consequences for people undergoing genetic testing. New Zealand law does not prohibit insurers from discriminating based on genetics, so a positive or inconclusive result may impact premiums and eligibility for health insurance and life insurance.35
For information on the follow-up and surveillance of a patient after curative-intent treatment for ovarian cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
 There is a B-QuiCK summary available for this topic
There is a B-QuiCK summary available for this topic





