Key practice points:
- Endometrial cancer is the predominant type of uterine cancer (95% of cases); uterine sarcomas make up ≤ 5% of all endometrial cancer diagnoses
- Endometrial cancer is primarily caused by excessive exposure to endogenous or exogenous oestrogen unopposed by progesterone. Significant risk factors include increasing age, obesity, tamoxifen use and Lynch syndrome (see main text for a full list). Pacific peoples have the highest incidence of endometrial cancer amongst any ethnic group.
- Most people with endometrial cancer are diagnosed when they are post-menopausal. Symptoms may include abnormal uterine bleeding (particularly post-menopausal, but also inter-menstrual or post-coital), abnormal vaginal discharge, unexplained weight loss, pelvic or abdominal pain or distension and urinary or bowel dysfunction.
- There are no screening programmes available or recommended for the early detection of endometrial cancer, so diagnosis relies on recognising and investigating suspicious symptoms
- Some genetic conditions increase the risk of endometrial cancer; check local HealthPathways to assess eligibility criteria for referral to genetic services. Females with Lynch syndrome should be referred to a gynaecologist for discussion about ongoing surveillance or risk-reducing strategies for endometrial cancer.
- The diagnostic workup of a patient with symptoms suggestive of endometrial cancer includes:
- A focused history, considering relevant risk factors
- A physical examination, including bimanual and speculum examination; with cervical screening if due or swabs for sexually transmitted infections (if indicated)
- Additional laboratory tests, depending on individual factors. These may include: full blood count, ferritin, liver function tests, coagulation tests, thyroid stimulating hormone, HbA1c, pregnancy test (if appropriate).
- Endometrial biopsy (pipelle), ideally performed in primary care, and referral for a pelvic ultrasound are generally recommended for patients with suspected endometrial cancer
- Referral to a gynaecologist for further assessment is indicated for patients with recurrent post-menopausal bleeding (or any post-menopausal bleeding if taking tamoxifen), and patients with abnormal findings on biopsy or ultrasound
N.B. The term “female” is used in this article to describe the biological sex of the patient population at risk for endometrial cancer. However, we acknowledge that this may not reflect the identity of all patients, which will include transgender boys or men, intersex and non-binary individuals.
For information on the follow-up and surveillance of a patient after curative-intent treatment for endometrial cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
Endometrial cancer (adenocarcinoma arising from the endometrium) is the main type of uterine cancer (95%); uterine sarcomas (arising from the myometrium or connective tissues that support the endometrium) occur rarely (≤ 5%).1 Data are limited but uterine sarcomas
are typically high grade and associated with a poor prognosis.2
Historically, endometrial cancers are further divided into Type I (endometrioid; driven by oestrogen) and Type II (non-endometrioid; non-oestrogen driven).3, 4 This classification was first proposed in the early 1980s5 and remains current practice in New Zealand. However, a new molecular classification system (The Cancer Genome Atlas [TCGA] endometrial cancer classification system) has been incorporated into some international guidelines, and is in the process of being implemented throughout New Zealand (for further information, see: “An overview of the pathogenesis of endometrial cancer”).6 As the majority of uterine cancer diagnoses are endometrial cancers, they are the most frequently studied and the focus of this article.
Endometrial cancer* is the most common gynaecological cancer with an average of 627 new diagnoses (17.1 per 100,000 females; from 2015 – 2020) and 136 related deaths (3.3 per 100,000 females; from 2015 – 2018)† each year in New Zealand;** in 2020 it was the fifth most common cancer in females.7 The risk of endometrial cancer increases with age and it primarily affects females who are post-menopausal with an average age at diagnosis of 60 – 65 years.8 This is reflected in New Zealand data where the highest incidence of endometrial cancer is among females aged 45 – 64 years.9
*Data in New Zealand are only available for uterine cancer overall; endometrial cancers represent approximately 95% of all uterine cancer diagnoses3
†Mortality data are available for 2019, but are preliminary so have not been included. Mortality data for 2020 are not yet available.
**There are differences in published data. For consistency across the gynaecological cancer series, incidence and mortality data has primarily been obtained from the publication – Cancer: Historical summary 1948 – 2020.
Compared with other gynaecological cancers, people with endometrial cancer are usually symptomatic, and therefore more commonly diagnosed when their cancer is at an early stage and still confined to the uterus.2, 10 The early detection and treatment of endometrial cancer is associated with a good prognosis and a five-year survival rate of up to 90%.2, 11
The overall incidence of endometrial cancer is increasing over time
The overall incidence of endometrial cancer is increasing both in New Zealand (Figure 1) and worldwide, which is thought to reflect a rise in the prevalence of risk factors, particularly obesity and an ageing population (see: “Risk factors for endometrial cancer”).10, 12 The incidence of endometrial cancer in New Zealand is similar to other comparable countries, e.g. Australia, United Kingdom.13
Although the risk of endometrial cancer increases with age, there has been an increase in the number of people diagnosed with endometrial cancer at a younger age, i.e. aged under 40 – 50 years.10, 14 In particular, the incidence is increasing in younger females who are obese and Pacific peoples.14, 15 Mortality rates in New Zealand have remained relatively consistent over time (Figure 1).

Figure 1. Age-standardised incidence and mortality rates (per 100,000 females) for uterine cancer between 2015 and 2018 in New Zealand.7 N.B. Data in New Zealand are only available for uterine cancer overall; endometrial cancers represent approximately 95% of all uterine cancer diagnoses.3 Incidence data are available up to 2020 but have not been included in this graph for comparative purposes. Mortality data are available for 2019, but are preliminary so have not been included. Mortality data for 2020 are not yet available.
There are significant ethnic disparities associated with endometrial cancer
- Māori and Pacific peoples have a higher incidence of endometrial cancer each year, compared to European/Other (Figure 2).16 They are also more likely to be diagnosed with endometrial cancer at a higher grade and have poorer survival.12, 17
- Pacific peoples have the highest incidence of endometrial cancer amongst any ethnic group and this is increasing more rapidly than in Māori and European populations.15 In 2019 (most recent ethnicity data available), the age-standardised incidence rate for Pacific peoples was 78.7 per 100,000 females, which was 2.8 times greater than the rate in Māori (27.8 per 100,000 females) and 6.0 times greater than in European/Other ethnic groups (13.1 per 100,000 females).16
- In a study published in 2009 it was estimated that 37% of endometrial cancers diagnosed in Māori and 48% in Pacific peoples were attributable to increased body mass index.18 As obesity rates continue to rise, it is likely that these numbers have increased further.
- There is a higher incidence of endometrial cancer among people living in low socioeconomic areas.12
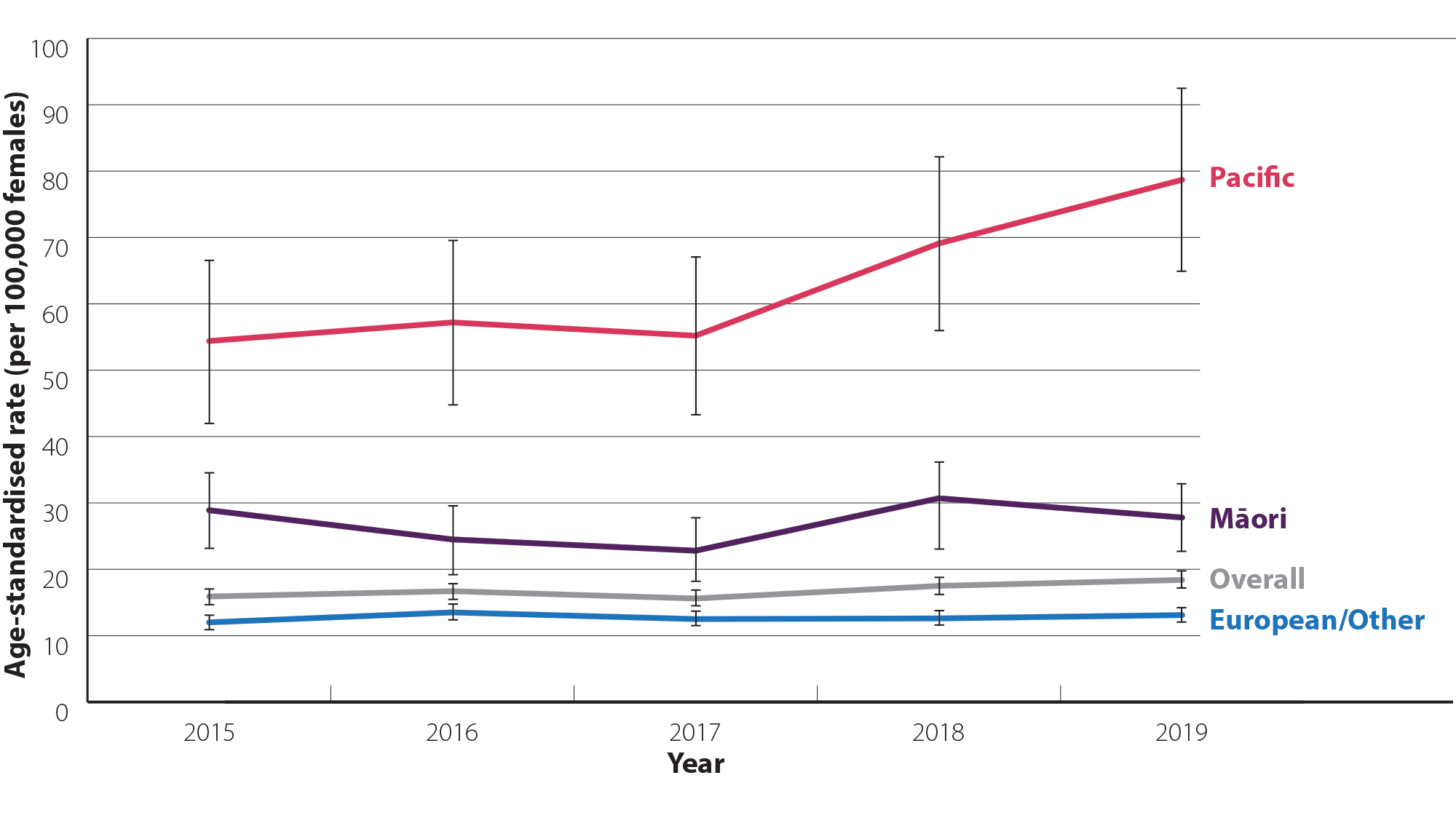
Figure 2. Age-standardised incidence rate (per 100,000 females; 95% confidence intervals)* for uterine cancer by ethnicity between 2015 and 2019 in New Zealand.16 N.B. Data in New Zealand are only available for uterine cancer overall; endometrial cancers represent approximately 95% of all uterine cancer diagnoses.3 Incidence data by ethnicity are not yet available for 2020.
* Confidence intervals have been included as Pacific peoples have a smaller sample size than other groups, so rate estimates are less stable (i.e. rates are more likely to vary from year-to-year due to chance)
An overview of the pathogenesis of endometrial cancer
Historically, endometrial cancer is categorised into Type I and Type II. The types differ in incidence, aetiology and prognosis; together they make up 95% of all uterine cancer diagnoses.3, 4 This classification system of endometrial cancer, dating from 1983,5 remains current practice in New Zealand, however, it is no longer routinely used internationally. A new molecular classification system (The Cancer Genome Atlas [TCGA] endometrial cancer classification system, based on three immunohistochemical markers and one molecular marker) has been incorporated into some international guidelines, and is in the process of being implemented in New Zealand.6
The historical classification system (Type I and II) has been useful in providing a conceptual framework for understanding the pathogenesis of endometrial cancer, however, it does not reflect the heterogeneity of these cancers, particularly those that are high-grade which may not fit into either of the two types.2, 4 The TCGA molecular classification system has been shown to overcome this and improve clinical outcomes by providing more detailed prognostic information, and has the potential to inform treatment decisions and possibly give rise to novel treatments.2, 4
The TCGA will take some time to be fully implemented in practice, therefore it is still of value to examine the current Type I/II classification system in more detail:
Type I endometrial cancer (endometrioid cancer) is the most common and accounts for approximately 80% of all endometrial cancers.2, 8 These tumours are usually diagnosed at an early stage with a favourable prognosis.2 Type I endometrial cancers are typically caused by excessive exposure to unopposed endogenous or exogenous oestrogen (see below), and are often preceded by pre-cancerous lesions (atypical endometrial hyperplasia).8, 10
The oestrogen theory of the development of Type I endometrial cancer
The endometrium undergoes cellular and structural changes in response to fluctuations of oestrogen and progesterone. During a normal menstrual cycle, oestrogen stimulates endometrial proliferation, while progesterone inhibits proliferation and stimulates differentiation in preparation for an embryo. Most endometrial cancers develop over time from sustained proliferation of the endometrium due to increased exposure to oestrogen, unopposed by progesterone.8, 10
Increased exposure to oestrogen can be due to endogenous factors, e.g. early menarche, late menopause, obesity, or exogenous factors, e.g. tamoxifen, use of an oestrogen-only menopausal hormone therapy (see: “Risk factors for endometrial cancer”).10
Type II endometrial cancer (or non-endometrioid cancer), e.g. serous, clear cell mucinous carcinomas and carcinosarcomas, represent the remaining proportion of endometrial cancers. The aetiology of Type II endometrial cancers is unclear as few studies are available; however, in contrast to Type I, they are less commonly oestrogen-dependent (although obesity remains linked as a risk factor in some cases) and have no known precursor lesions.8, 14 Type II endometrial cancers are more often diagnosed in older females who have had children (likely due to endometrial atrophy associated with increasing age), and are also often at a more advanced stage and associated with a poorer prognosis.2, 8
Most endometrial cancers are aetiologically linked to excessive exposure to endogenous or exogenous oestrogen unopposed by progesterone, with the most significant risk factors being increasing age (≥ 55 years) and obesity.11, 10 Some people may also have a genetic predisposition; familial cancer syndromes are estimated to cause up to 5% of all endometrial cancers.12 Table 1 details additional risk factors.
- Obesity. The lifetime risk of endometrial cancer in females is 3% but with every five unit increase in a person’s body mass index, the relative risk increases by more than 50%.10 Excess body weight is associated with high levels of endogenous oestrogen due to the conversion of androgens to oestrogen in adipose tissue.11 Higher oestrogen levels then lead to greater stimulation of the endometrium to proliferate and therefore, an increased risk of endometrial cancer.11
Table 1. Risk factors for endometrial cancer.3, 8, 10, 11
| Endogenous oestrogen |
Exogenous oestrogen |
- Increased cumulative exposure to oestrogen due to increasing age, obesity, early menarche (< 12 years), late menopause (≥ 55 years) or nulliparity
- Anovulation, e.g. due to polycystic ovary syndrome
- Oestrogen secreting tumour, e.g. ovarian granulosa cell tumours
|
- Oestrogen-only menopausal hormone therapy
- Tamoxifen use in people aged ≥ 50 years
|
| Family history and genetics |
Lifestyle and medical factors |
- Family history of endometrial, ovarian or colorectal cancer (check local HealthPathways to assess eligibility criteria for referral to genetic services)
- Lynch syndrome (see: “Surveillance for endometrial cancer in people with Lynch syndrome”)
- Cowden syndrome (germline mutations in PTEN – a tumour suppressor gene)
- Somatic mutations in PTEN gene
- There is a reported association between a personal history of breast cancer and an increased risk of endometrial cancer; further study is required
N.B. The association between BRCA1 and BRCA2 mutations and the risk of endometrial cancer is unclear. |
- Obesity, type 2 diabetes, hypertension, dyslipidaemia
- Endometrial hyperplasia
- Polyps in post-menopausal females or females with abnormal uterine bleeding
|
Some people may be able to modify their risk of endometrial cancer
Many cases of endometrial cancer cannot be prevented, however, there is evidence that even slight changes to certain predisposing factors, e.g. obesity, can significantly reduce the risk of endometrial cancer.8 While an estimated six out of every ten diagnoses of endometrial cancer are attributed to obesity, more than half of all people diagnosed are not aware of this risk.17 Given the general gap in awareness regarding the modifiable risk factors for endometrial cancer, consider whether patients might benefit from a discussion on risk-reducing measures (below), if appropriate.8
Protective factors include:2, 3, 8, 10
- A healthy diet and physical activity to maintain a healthy body weight. Studies have shown a significant reduction in endometrial cancer risk (up to 81%) in females who were obese who had bariatric surgery
and maintained a normal body weight.20
- High lifetime exposure to endogenous or exogenous progesterone. Progesterone opposes the risk of excessive oestrogen. The following factors have been shown to reduce the risk of endometrial cancer:
- Regular menstrual cycles
- Pregnancy
- Breastfeeding
- Prolonged combined oral contraceptive use. Risk reduces with longer duration of use and persists for several decades after cessation.
- Combined oestrogen and progestogen menopausal hormone therapy
- Use of a progestin-releasing intrauterine system, e.g. Mirena*. This may be an effective risk reduction strategy for people at high risk of endometrial cancer, e.g. those with obesity.21
*There is no evidence of an association between copper intrauterine devices and endometrial cancer8
Surveillance for endometrial cancer in people with Lynch syndrome

Lynch syndrome (previously known as hereditary non-polyposis colorectal cancer) is an inherited condition that results from a germline mutation in one of the four DNA mismatch repair genes, MLH1, MSH2, MSH6 or PMS2. It is associated with a significantly increased risk of developing colorectal, endometrial and ovarian cancers, among others.1, 10
People with Lynch syndrome have a lifetime risk of endometrial cancer of 40 – 60% and are more likely to be diagnosed with endometrial cancer at a younger age.14 Lynch syndrome accounts for approximately 3% of endometrial cancers and 9% of all cases diagnosed in people aged < 50 years.11, 14
In contrast to colorectal cancer screening in people with Lynch syndrome, there is currently no high-quality evidence that screening people for endometrial cancer improves survival outcomes.4 Despite this, some international societies, based on expert opinion, recommend offering screening for females who have not completed childbearing, with pelvic/transvaginal ultrasound*, hysteroscopy and/or endometrial biopsy, every one to two years from age 30 – 35 years after counselling about the risks, benefits and limitations of screening.2, 4
Risk-reducing surgery, i.e. hysterectomy with or without bilateral salpingo-oophorectomy (due to concurrent risk of ovarian cancer), may be considered for females with Lynch syndrome after childbearing is complete (or not desired), ideally between the ages of 35 – 50 years, depending on the specific germline mutation.4, 22
*Ultrasound is not generally recommended as a screening tool in people who are pre-menopausal2
A pragmatic approach in New Zealand may be to refer patients with Lynch syndrome to a gynaecologist for discussion about ongoing surveillance and management including risk-reducing surgery, and to recommend general ways to improve lifestyle-related risk factors. This may include discussion about maintaining a healthy body weight, being physically active, reducing alcohol intake, smoking cessation, use of the combined oral contraceptive pill or progestin-releasing intrauterine system if contraception is required and avoiding the use of oestrogen-only menopausal hormone therapy.10 Encourage patients to present to primary care early if they have any new or concerning symptoms.4
There are no screening programmes for the early detection of endometrial cancer in the general population or for high-risk groups.4 ,10 However, symptoms of endometrial cancer usually appear early, and approximately 95% of people diagnosed with endometrial cancer are symptomatic.1 Therefore, prompt recognition and investigation of suspicious symptoms is important to achieve optimal survival outcomes.
Abnormal bleeding (usually post-menopausal bleeding) is one of the most commonly reported symptoms in people with endometrial cancer.1, 2 The presence of multiple relevant symptoms and signs (see below), particularly in conjunction with risk factors should increase suspicion of endometrial cancer.3
Symptoms or signs of endometrial cancer may include:3, 10, 11
- Abnormal bleeding including post-menopausal, inter-menstrual and post-coital bleeding
- Abnormal vaginal discharge
- Unexplained weight loss
- Pelvic pain
- Abdominal pain or distension
- Urinary or bowel dysfunction
Although the cause of post-menopausal bleeding is usually benign, e.g. vaginal atrophy, polyps, it is important not to miss the 5 – 10% of cases where endometrial cancer is the underlying cause.10, 23 Some clinicians practise by the rule that “post-menopausal bleeding is endometrial cancer until proven otherwise”.
For further information on post-menopausal bleeding, see: bpac.org.nz/2019/bleeding.aspx
Consider how education about the key symptoms of endometrial cancer might be incorporated during relevant discussions, e.g. about menopause or menopausal hormone therapy, to minimise delayed presentation and to improve the likelihood of early detection.10, 19
Diagnosing endometrial cancer in pre-menopausal females
The diagnosis of endometrial cancer in females who are pre-menopausal is often difficult as symptoms tend to be non-specific and commonly related to another cause, e.g. heavy, prolonged or inter-menstrual bleeding.10 Given the low risk of endometrial cancer in this population, the decision of whether to investigate for this further should be guided by relevant risk factors in the patient history, e.g. family history of cancer syndromes, obesity, ethnicity (e.g. Pasifika), and clinical suspicion from examination findings.2, 10
Begin initial workup with a focused history and physical examination
When assessing a patient with symptoms suggestive of endometrial cancer, begin by taking a history to identify any relevant risk factors such as family history, ethnicity (e.g. Pasifika), or factors that would result in excess exposure to unopposed oestrogen, e.g. tamoxifen use, oestrogen-only menopausal hormone therapy, obesity.3, 4
If abnormal uterine bleeding is reported, determine the nature of the bleeding, including frequency, duration, quantity and precipitating factors, and ask about any associated symptoms such as pain or discomfort, fever and changes in bladder or bowel function. Consider potential causes, e.g. polycystic ovary syndrome, hypothyroidism, medicines such as anticoagulants, antidepressants or hormonal contraceptives. N.B. People newly initiated on menopausal hormone therapy may experience bleeding within the first three to six months of use and do not usually require further investigation. If bleeding occurs or persists after six months of continuous use, refer the patient for further assessment, e.g. for an ultrasound; manage according to the results.
Perform an abdominal and pelvic examination, including bimanual and speculum examination to identify any abnormalities and to exclude potential local causes of the patients symptoms.4, 23 Physical examination findings are often normal in patients with early stage endometrial cancer but the presence of an enlarged uterus on bimanual examination or palpable inguinal or supraclavicular nodes should increase suspicion of more advanced cancer.1
Cervical screening should be offered to patients who are due. Further investigation is required if endometrial cells are detected by cervical cytology in people who are post-menopausal due to an increased risk (3 – 5%) of endometrial cancer.4 Also swab for sexually transmitted infections, e.g. chlamydia, gonorrhoea, if indicated.
There are no specific laboratory tests (other than analysis of biopsy if performed) for the diagnosis of endometrial cancer, but they may be requested (as indicated) to assess other aspects of the patient’s health and to identify any other potential underlying cause of the symptoms, e.g. hypothyroidism. Most laboratory test results will be normal except in patients with significant bleeding, who may be anaemic. Tests may include:19, 23, 24
- Full blood count
- Ferritin
- Liver function tests
- Coagulation tests (prothrombin time, international normalised ratio, activated partial thromboplastin time and fibrinogen)
- Thyroid stimulating hormone
- HbA1c
- Urine pregnancy test or serum hCG (if appropriate)
Refer patients with suspicion of endometrial cancer for a pelvic ultrasound and endometrial biopsy (pipelle)
A pelvic ultrasound* and endometrial biopsy (pipelle) are generally recommended for patients with suspected endometrial cancer.23 The optimal order of investigations varies depending on access and service capacity, clinical judgement and patient preference.4
*Pelvic ultrasound for patients with suspected endometrial cancer usually includes a transvaginal ultrasound. It is recommended that this is discussed with patients prior to the referral so they know what to expect and because some patients may decline.
Referral for a pelvic ultrasound is most commonly requested first to exclude structural causes of the patient’s symptoms, e.g. polyps, and usually includes a transvaginal ultrasound to measure endometrial thickness.4, 23 Patients with endometrial pathology or a thickened endometrium (e.g. ≥ 5 mm; check local HealthPathways for regional guidance) on ultrasound require follow-up with a pipelle biopsy.10, 23 However, wait times for a pelvic ultrasound can be long in some regions; if wait times are likely to exceed two weeks or if there is difficulty for the patient to access care, the pipelle biopsy should be performed first.
A pipelle biopsy is used to take a sample of the endometrial tissue and has high diagnostic accuracy for detecting endometrial cancer.23 Ideally, the pipelle biopsy should be performed in primary care, but resource limitations may mean that patients require timely referral to another general practice colleague or other provider. Prior to the biopsy procedure, provide patients with an information leaflet: www.healthinfo.org.nz/patientinfo/27989.pdf
N.B. Pipelle biopsies are contraindicated during pregnancy. Cautions include the presence of cervicitis or endometritis, pelvic inflammatory disease or other infection, coagulation disorders, anticoagulant use; check HealthPathways for a complete list of cautions. It is difficult to perform a pipelle biopsy on a patient with cervical stenosis, referral to a gynaecologist is usually required.
-
If a patient is taking tamoxifen and reports post-menopausal bleeding, organise a pipelle biopsy and request gynaecology referral for consideration of a hysteroscopy* (or request hysteroscopy directly, if available)4 – annotate referral with “high suspicion of cancer”
*Both endometrial biopsy and hysteroscopy are required in these patients as pelvic ultrasound is not sensitive nor specific for hyperplasia due to the tamoxifen-induced changes to the endometrium
N.B. Guidance may differ between regions; check local HealthPathways for specific advice.
If endometrial thickness from transvaginal ultrasound is < 8 mm and the results from the pipelle biopsy are normal, initiate treatment for atrophic vaginitis. Place a recall to review the patient within two months; if bleeding persists, refer the patient to a gynaecologist for further assessment.
For information on the treatment of a patient with atrophic vaginitis, see: bpac.org.nz/bpj/2014/september/vulvovaginal.aspx
Referral to gynaecology is generally indicated if:
- Pelvic/transvaginal ultrasound shows fibroids causing significant uterine enlargement or cavity distortion, endometrial polyp(s), high-risk features or other abnormalities such as cystic spaces
- The endometrial thickness is:
- < 5 mm and there is persistent bleeding despite treatment for atrophia
- 5 – 8 mm and the pipelle biopsy sample is inadequate
- > 8 mm, irrespective of the results from pipelle biopsy
- The pipelle biopsy:
- Is indicated but cannot be performed in primary care
- Is inadequate, insufficient or limited
- Shows hyperplasia without atypia
- Urgently refer patients to a gynaecologist if the results show hyperplasia with atypia or endometrial carcinoma. Annotate referral with “high suspicion of cancer”.
International guidelines recommend that people diagnosed with endometrial cancer should be tested for Lynch syndrome.4, 25 This test is performed by pathologists in New Zealand on the tumour using mismatch repair (MMR) immunohistochemistry. Referral for formal genetic (germline) testing is usually organised after abnormal immunohistochemistry results, e.g. MMR deficiency, or a significant family history of endometrial or colorectal cancer.3, 25 Further information about genetic testing, including details about the referral process and cancer assessments is available through Genetic Health Service New Zealand, or some local HealthPathways.
For information on the follow-up and surveillance of a patient after curative-intent treatment for endometrial cancer, see: https://bpac.org.nz/2023/gynaecological-cancers.aspx.
 There is a B-QuiCK summary available for this topic
There is a B-QuiCK summary available for this topic





