Key practice points:
- Diverticulosis (asymptomatic diverticula) is often an incidental finding; prevalence increases with age
- Approximately 4% of people with diverticulosis will develop diverticulitis
- Factors that increase the risk of developing diverticulitis include Western diets (often high in red meat and refined grains, and low in fibre), central obesity, smoking, some medicines, e.g. NSAIDs or opioids, and genetics
- Clinical features of diverticulitis, e.g. abdominal pain and changes in bowel habit, are non-specific and differential diagnoses include appendicitis, inflammatory bowel disease, irritable bowel syndrome and colorectal cancer
- International guidelines recommend a CT scan with contrast to diagnose acute diverticulitis, however, limited access and availability of appropriate imaging in New Zealand means a more pragmatic approach is required:
- Patients with red flag symptoms indicative of complicated diverticulitis, e.g. abscess, perforation, obstruction or fistula, significant immune suppression or relevant uncontrolled co-morbidities likely to worsen their condition, e.g. diabetes, liver or renal disease, require referral to secondary care (where a CT scan can be performed to confirm the diagnosis)
- For patients with less severe symptoms, a clinical diagnosis of uncomplicated diverticulitis can be made after reasonable exclusion of other causes, and conservative treatment initiated in the community, including paracetamol (NSAIDs or weak opioids can be considered if no contraindications). Patients should be ideally followed up in 48 hours, or earlier depending on their clinical condition.
- While conventional advice has recommended short-term diet modification for patients with acute uncomplicated diverticulitis, i.e. two to three days of clear liquids before slowly reintroducing dietary fibre, there is a lack of clinical evidence to support this. If tolerated, an unmodified diet may be more appropriate. Consider patient preference.
- Antibiotics are no longer routinely recommended for most patients with suspected acute uncomplicated diverticulitis; oral antibiotics may be considered for some patients who are at higher risk of complications (e.g. due to co-morbidities), but who do not meet criteria for secondary care referral
- Antibiotics can also be considered for patients managed conservatively who do not show improvement within 48 hours of their first presentation
- Advise patients who have recovered from acute diverticulitis to gradually introduce more dietary fibre to their diet and avoid NSAIDs to reduce the risk of future episodes
- Subsequent referral for colonoscopy to rule out colorectal cancer is recommended for patients with CT-proven complicated diverticulitis
Glossary of terms
Diverticulum – pouch-like protrusion that forms at a weak point of the gastrointestinal wall (diverticula plural)1
Diverticulosis – the presence of diverticula2
Uncomplicated diverticulitis – acute or long-term inflammation of the gastrointestinal wall associated with diverticula but without the complications below2
Complicated diverticulitis – diverticulitis with either abscess, fistula formation, perforation of the intestinal wall, peritonitis or bowel obstruction2
Diverticular disease – symptomatic diverticulosis including diverticulitis and its associated complications3
Clear liquid diet – consumption of only transparent liquids, e.g. water, clear soups and juices, electrolyte drinks, to reduce the amount of stool in the colon and therefore stress on the gastrointestinal tract. Clear liquid diets are only appropriate for short periods due to insufficient calories and nutrition.
Low-fibre diet – consumption of refined grains, e.g. white bread and rice, and the avoidance of dietary fibre, e.g. fruit and vegetable skins/seeds, to reduce the quantity of stool passing through the colon.4 Low-fibre diets typically recommend < 10 g of dietary fibre per day.4, 5
N.B A low residue diet encompasses a reduction in fibre but as all foods produce gastrointestinal residue to some degree and there is no method to quantify this, the term low-fibre diet is now favoured.4, 5
A diverticulum is a pouch-like protrusion that forms at a weak point in the gastrointestinal wall.6 Diverticula can be considered either pseudo-diverticula or true diverticula.1 Pseudo-diverticula are pockets that only involve mucosa and submucosa, and occur at specific points where afferent arterioles (vasa recta) penetrate the smooth muscle layer of the colon.1 Pseudo- or “false” diverticula are most often found in Western populations.1 In contrast, true diverticula also incorporate the muscle layer of the gastrointestinal tract as well as the mucosa and submucosa, and are more prevalent in people of Asian ethnicity.1
Diverticula are most commonly observed in the colon - the sigmoid and descending colon in Western populations and the ascending colon in people of Asian ethnicity, although they can occur at any point along the gastrointestinal tract, i.e. oesophagus, stomach and the small intestine.1 Patients are diagnosed with diverticulosis if diverticula are found to be present, e.g. incidentally during a colonoscopy or computed tomography (CT) scan. Most patients with diverticulosis are asymptomatic; approximately 4%, however, will develop acute or long-term inflammation of the colon wall associated with diverticula, i.e. diverticulitis.7 Diverticular disease is a general term that encompasses symptomatic diverticulosis (without inflammation), diverticulitis and other complications associated with the presence of diverticula, e.g. abscess, gastrointestinal bleeding, fistula formation, perforation of the intestinal wall, peritonitis or bowel obstruction (Figure 1).6, 8
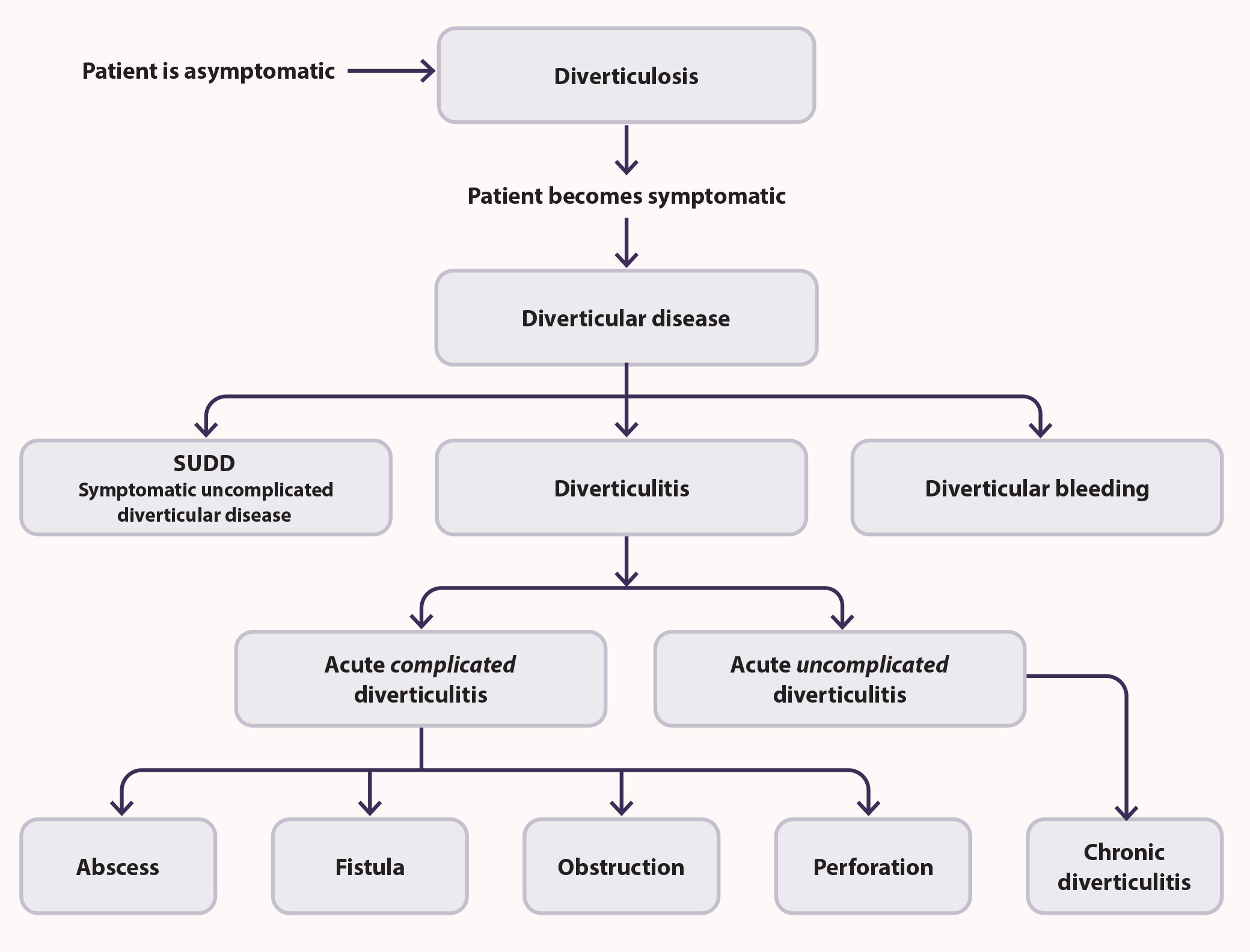
Figure 1. Diverticular disease encompasses a range of clinical conditions that develop in patients with diverticulosis.6, 10
The epidemiological distribution of diverticulitis is changing
Estimating the prevalence of diverticulosis is difficult as most people remain asymptomatic and diagnosis generally occurs during unrelated investigations.13 Rates of diverticulosis increase with age.5 International data show that colonic diverticula are present in more than 60% of people aged over 80 years and more than 50% aged over 60 years.5 A similar distribution is seen in New Zealand; a 2021 retrospective review of patient records following CT imaging for suspected urolithiasis found that 48% of participants aged 50 – 59 years and 64% of those aged 70 years or older had diverticulosis.13 In contrast, diverticulosis was found in only 17% of patients aged 18 – 29 years.13
Globally, diverticulosis is far more common in Western countries and previous studies have reported a prevalence of between 5 and 45%.5 In people who have diverticulosis, left-sided colonic diverticula are found in more than 90% of people of European ethnicity, whereas 70 – 74% of people of African or Asian ethnicity will have right-sided diverticula, i.e. in the ascending colon.14 However, increasing rates of left-sided diverticula are being reported in Asian countries, and could be related to the incorporation of low-fibre Western diets.5 The New Zealand-based study identified diverticulosis in 42% of participants overall.13 The prevalence of right-sided diverticulosis was higher in people of Asian ethnicity (14.5%), compared to New Zealand European (9.1%), Māori (7.7%) and Pacific peoples (9.4%).13
Only a small number of people with diverticulosis will progress to diverticulitis, estimated to be around 4%, and in most people this will occur at an older age.7, 15 In New Zealand, the age at which patients are hospitalised* with diverticulitis differs by ethnicity; Māori (56.2 years), Pacific peoples (58.4 years) and people of Asian ethnicity (58.9 years) develop diverticular disease and present to hospital at a younger age than New Zealand Europeans (65.8 years).16
Although diverticulitis is more likely to be diagnosed in older people, younger males are being hospitalised with diverticular disease at far higher rates than previously reported.5 New Zealand data show that between 2005 and 2015, there was nearly a 100% increase in rates of hospital admissions for diverticular disease in males aged 30 – 54 years (3.1 per 10,000 person-years in 2005 to 5.9 per 10,000 person-years in 2015).15 Similar trends in the rising rates of diverticulitis in males aged under 40 years have been reported overseas.17, 18
*Patients with severe acute diverticulitis either present to the emergency department or are referred to hospital by their general practitioner. Hospital records can therefore only provide an estimate of rates of more severe disease.
The aetiology of diverticulosis is not fully understood and likely multifactorial, including increasing age, dysfunction of the colon wall, a lack of dietary fibre and genetics.2, 6, 10 A commonly held theory is that a lack of dietary fibre can cause constipation, which leads to luminal pressure build-up in the colon causing pouch-like protrusions at weak points in the gastrointestinal wall.1, 9 However, more recent studies have cast doubt on this theory (although a low-fibre diet is associated with diverticulitis).6 Connective tissue dysfunction, e.g. in people with Marfan or Ehlers-Danlos syndromes, polycystic kidney disease or rectal prolapse, has also been associated with a higher risk of developing diverticula.19
The exact causes of diverticulitis are also unclear. Current theories include chronic inflammation, alterations in gut microbiome, defects in gastrointestinal smooth muscle and genetic causes.3 Previously, the blockage of diverticula by non-digestible food, e.g. a seed, was thought to result in inflammation, microperforation and local infection by anaerobic bacteria, e.g. Bacteriodes or Peptostreptococcus.3, 20 In more severe situations, this can result in abscess formation or macroperforation of the bowel wall.3 It is now accepted that obstruction is rare and diets high in nuts, seeds and corn do not increase this risk of developing diverticulitis, suggesting there are other mechanisms involved.3 The place of bacterial infection in the pathogenesis of diverticulitis is also being questioned as most cases of mild, uncomplicated diverticulitis resolve spontaneously, and antibiotics do not speed up recovery or reduce the risk of complications (see: “Antibiotics are no longer the cornerstone of management for acute uncomplicated diverticulitis“).20
For people who already have diverticulosis, risk factors that influence the development of acute diverticulitis include:
- Increasing age2
- Western diets, high in red meat and refined grains and low in fibre3
- Obesity, specifically central obesity3, 18
- Smoking3, 18
- Medicines including NSAIDs, opioids and corticosteroids3, 18
- Family history and genetics (reportedly account for 40 – 50% of diverticular disease)9
Other conditions associated with diverticular disease
Diverticular haemorrhage – painless lower gastrointestinal bleeding can be associated with diverticula.1, 9 Bleeding from diverticula typically involves larger volumes of either bright red blood (from diverticula in the sigmoid colon or rectum) or darker melaena (associated with right-sided diverticula).5 Diverticular bleeding is more commonly seen in males and people of Asian ethnicity.1, 5 Other risk factors for diverticular haemorrhage include use of NSAIDs, physical inactivity, obesity, diabetes and hypertension.1, 9 Spontaneous haemostasis without treatment can occur,1 although often patients require referral to hospital for urgent investigations and control of bleeding.10
Best Practice Tip: Strongly consider colorectal malignancy in patients presenting with symptoms suggestive of acute diverticulitis alongside bleeding or anaemia.11
Symptomatic uncomplicated diverticular disease (SUDD) – gastrointestinal symptoms, e.g. bloating, abdominal pain, changes in bowel habits, occurring in patients with diverticulosis but with no evidence of local inflammation or bleeding.1, 6 Significant symptom crossover exists between SUDD and irritable bowel syndrome (IBS) and there is debate regarding its status as a standalone condition or simply the co-existence of diverticulosis alongside IBS.1, 4
Segmental colitis associated with diverticulosis (SCAD) – specific inflammation of the sigmoidal colon mucosa between diverticula (which are typically uninvolved).12 SCAD differs from other forms of inflammatory bowel disease as it is predominantly diagnosed in older males, i.e. aged over 50 years, the rectal mucosa is typically unaffected and it is responsive to pharmacological treatments, e.g. mesalazine.12
Managing diverticulosis

A diagnosis of diverticulosis is often incidental following a radiological examination or colonoscopy.1 In most people, diverticulosis does not require specific treatment and they will often remain asymptomatic.1 Guidelines recommend general lifestyle modification to prevent or slow the progression to diverticulitis.21 Maintaining a healthy body weight and regular exercise have been shown to decrease diverticulitis risk.3
Discussions with the patient should involve:21, 22
- Eating a balanced diet high in fruits, vegetables and whole grains and limiting red meat
- Importance of adequate hydration
- “Bowel health”, i.e. keep stools soft and bulky via dietary methods above; bulk-forming laxatives may be considered in patients with constipation who are intolerant to increasing fibre in their diet
- Weight loss and increased physical activity (if required)
- Smoking cessation (if applicable)
- Avoiding use of over-the-counter NSAIDs
The symptoms of diverticulitis are non-specific, e.g. intermittent abdominal pain and other general gastrointestinal symptoms. In some cases, patients will know they have diverticulosis (e.g. due to a previous colonoscopy or abdominal CT scan), or have already had diverticulitis, in which case a clinical diagnosis of acute diverticulitis in the presence of characteristic symptoms is often straightforward. In other cases, where there is no relevant history, diverticulitis will be one of several differential diagnoses.
A pragmatic approach to acute diverticulitis in primary care
Although internationally, contrast CT is the gold standard for a formal diagnosis of diverticulitis,18, 21, 23 most primary care clinicians will not have direct access to imaging.24 Therefore, a pragmatic approach to making a clinical diagnosis of acute diverticulitis is required, especially given many patients with acute, uncomplicated diverticulitis will follow a benign course, not require hospitalisation and rarely experience severe complications.10
For patients with symptoms indicative of diverticulitis in primary care, clinicians should:
- Consider if there is a history of diverticulosis (and diverticulitis)
- Perform a physical examination and consider relevant investigations, e.g. full blood count, CRP and urinalysis
- Assess for red flag symptoms or significant co-morbidities (see below)
- Consider other potential causes of symptoms
- Make a working diagnosis of diverticulitis and decide if the patient is well enough to be managed in the community or if they require referral to secondary care
- Initiate conservative treatment if community management is appropriate, i.e. analgesia
- Oral antibiotics are not required at initial presentation for most patients with suspected acute uncomplicated diverticulitis and mild symptoms
- However, without access to imaging, clinicians will need to use their judgement to determine which patients may benefit from antibiotics, e.g. those who are at higher risk of complications, taking certain medicines (such as corticosteroids, anticoagulants) or systemically unwell but not yet meeting local criteria for hospital referral
- Ideally, reassess the patient after 48 hours (or sooner if indicated); oral antibiotics can be considered again at this point if the patient’s condition has not improved but they still do not require secondary care referral
- Refer the patient to secondary care if red flag symptoms develop during community management or if there is an inadequate response to treatment following the initiation of oral antibiotics
Other considerations for referral to secondary care relate to the patient’s ability to self-manage and make a full recovery. Patients who are unable to tolerate adequate oral intake, are frail or unable to manage safely at home may not be appropriate for community management. Whereas some patients with significant, but well managed co-morbidities and adequate support at home may not necessarily require referral to secondary care and can remain in the community. Clinical judgement is therefore required to assess a patient’s risk of developing complicated diverticulitis and appropriately triaging them.
Clinical features of acute uncomplicated diverticulitis are non-specific
Patients with acute diverticulitis will most often present with new-onset abdominal pain, usually in the lower left quadrant, that is described as constant and worsening with movement.2, 25 However, do not rule out diverticulitis in patients presenting with right-sided abdominal pain, especially those of Asian or African ethnicity.14, 21 Recent changes in bowel habits, e.g. constipation or diarrhoea, are commonly reported, although rectal bleeding is rare in acute diverticulitis.2, 11 Other non-specific gastrointestinal symptoms may include nausea, anorexia, bloating and flatulence.2, 25 Patients may also have a low grade fever.7
 Red flag symptoms and signs suggesting complicated diverticulitis
Red flag symptoms and signs suggesting complicated diverticulitis
Patients who present with any of the following symptoms and signs are at risk of severe outcomes and require urgent referral to secondary care:2, 21
- Sepsis, e.g. fever, pallor, systolic hypotension, tachycardia, elevated respiratory rate, altered mental state, reduced urine output
- Intra-abdominal abscess, e.g. palpable abdominal mass or peri-rectal fullness on rectal examination, severe pain, fever, abdominal rigidity and voluntary guarding
- Intestinal obstruction, e.g. severe pain, vomiting, constipation (or obstipation*) and abdominal distention
- Bowel perforation or peritonitis, e.g. severe pain, fever, abdominal rigidity and voluntary guarding
- Evidence of a fistula from the colon to the bladder (e.g. faecaluria, pneumaturia, pyuria) or vagina (e.g. passage of gas or faeces through the vagina)
*No passage of stool or flatus
A clinical diagnosis of diverticulitis can be made from patient history, a physical examination and laboratory testing
Ask about/consider:26
- Pain characteristics such as location, time course and exacerbating or relieving factors
- Any changes in bowel habits, e.g. constipation, diarrhoea, blood in stool and how long they have been occurring
- Any systemic symptoms, e.g. fever, chills, and when they started
- Urinary symptoms (due to the proximity of the bladder to the sigmoid colon)2
- Use of medicines associated with the development of diverticulitis or other conditions of the colon3, 27
- Diverticulitis, e.g. NSAIDs, corticosteroids, opioids
- Ischaemic colitis, e.g. combined oral contraceptives*
- Pseudo-obstruction, e.g. opioids
- Infectious colitis, e.g. recent antibiotic use, corticosteroids
- Previous episodes of diverticulitis (or a diagnosis of diverticulosis confirmed with imaging or colonoscopy)
- History of gastrointestinal problems or other significant co-morbidities
- Previous abdominal surgeries which may be a source of symptoms, e.g. abdominal adhesions following appendix or hernia surgery
- Family history of diverticulosis, diverticulitis, colorectal cancer, polyps or inflammatory bowel disease
*Oral contraceptives containing high doses of oestrogen and progesterone can promote hypercoagulability and result in ischaemic colitis
Physical examination should include temperature, blood pressure, heart rate and depending on the patient’s clinical condition, an assessment of dehydration.2 Palpate the abdomen to determine degree of tenderness, location, distension and presence of any guarding, rebound tenderness, rigidity or masses.2 Consider whether rectal or pelvic examination is required, i.e. to identify benign anal causes (e.g. haemorrhoids, anal fissures) or gynaecological causes (e.g. endometriosis) of pain.2
There are no specific laboratory investigations for diverticular disease, however, some tests may be useful for assessing disease severity in an unwell patient, e.g. CRP and white blood cell count, or when considering differential diagnoses.
Relevant investigations for patients presenting with abdominal pain may include:2, 26
- Full blood count
- Mild leukocytosis is suggestive of acute uncomplicated diverticulitis (but a normal leukocyte level does not rule diverticulitis out)
- Clinically significant leukocytosis may indicate complicated diverticulitis
- Anaemia could support a diagnosis of gastrointestinal bleeding and justify referral for a colonoscopy in some clinical situations
- CRP
- A CRP level of 5 – 50 mg/L in conjunction with associated gastrointestinal symptoms is suggestive of acute uncomplicated diverticulitis, compared to higher levels, e.g. > 140 mg/L, which are more likely to be associated with complications, e.g. perforation or abscess, and the patient requires urgent referral to secondary care1, 23
- Creatinine
- Consider urgent secondary care referral in patients with newly elevated serum creatinine, e.g. due to dehydration, sepsis, acute kidney injury
- LFTs
- Biliary causes of abdominal pain, e.g. acute cholecystitis, choledocholithiasis and cholangitis
- Acute hepatitis
- Urinalysis and culture (if indicated)
- Urine pregnancy test (if appropriate)
- Stool culture
- To exclude faecal pathogens if diarrhoea is a prominent feature, e.g. Clostridium difficile or Giardia2
Differential diagnosis
Acute uncomplicated diverticulitis can be difficult to differentiate from other gastrointestinal conditions.1 A wide range of possible diagnoses should be considered including:2
- Irritable bowel syndrome
- Inflammatory bowel disease, e.g. Crohn’s disease or ulcerative colitis
- Appendicitis (in patients with right-sided abdominal pain)*
- Ischaemic or infectious colitis
- Faecal impaction
- Bowel perforation
- Colorectal cancer (or other abdominal cancers)
- Gynaecological conditions, e.g. endometriosis, pelvic inflammatory disease, ovarian torsion
- Urological causes, e.g. renal colic, cystitis, pyelonephritis
*Some medical centres may have access to a point of care ultrasound to aid diagnosis of appendicitis
 Patients presenting with severe abdominal pain or significant rectal bleeding require urgent referral to secondary care.
Patients presenting with severe abdominal pain or significant rectal bleeding require urgent referral to secondary care.
For further information on these differential diagnoses, see:
Identifying patients who could progress to complicated diverticulitis
Approximately 5% of patients with diverticulitis will develop complications such as perforation or abscess, usually within ten days of their initial presentation (but can be up to three months later).28 Patients who have had previous uncomplicated diverticulitis are less likely to develop complications in subsequent episodes.25
Potential predictors for progression to complicated diverticulitis measurable in primary care include:23, 28
- Symptoms persisting for longer than five days
- Vomiting
- Significantly elevated CRP, i.e. > 140 mg/L
- Leukocytosis, i.e. > 13.5 × 109 cells/L
Patients with these symptoms or signs may require earlier follow-up or a lower threshold for urgent referral to secondary care.
The appropriate level of intervention for patients with acute diverticulitis is determined by their clinical condition and co-morbidities.1, 2 The presence of severe symptoms or other red flags are clear indications that a patient requires management in secondary care. Ideally all other patients with mild-moderate symptoms and no red flags who present to primary care with suspected diverticulitis should be managed in the community. However, in practice, external factors that influence a patient’s ability to manage their own condition and recover without intervention must be considered.
Patients with uncomplicated acute diverticulitis can usually be managed in the community
Referral to hospital is not usually required for patients with acute uncomplicated diverticulitis and no risk factors, unless they deteriorate or do not improve.2 Most recent international guidelines recommend conservative outpatient management, where appropriate; previous management strategies, e.g. antibiotic treatment for all patients with suspected diverticulitis, lacked clinical evidence.21, 23
Home management is usually not appropriate for patients with:2
- Symptoms suggestive of complicated diverticulitis or systemic infection, e.g. peritonitis or sepsis (see: “Red flag symptoms and signs suggesting complicated diverticulitis”)
- Significant or uncontrolled co-morbidities, e.g. diabetes, end-stage liver or renal disease, immune suppression or other risk factors, e.g. pregnancy, older age or frailty
- No support at home (or who are unable to independently seek medical attention if symptoms do not improve)
- Difficulty tolerating oral fluids
- Difficulty controlling pain
An unrestricted diet is recommended, however, consider patient preference
If tolerated, patients should continue with their normal diet during an acute episode of diverticulitis.10 This recommendation may represent a change in practice for many clinicians given that conventional advice has been that patients follow a clear liquid diet during an acute episode of diverticulitis.2, 7, 25 The basis for this change is the increasing evidence that diet does not significantly affect patient outcomes, for example a 2017 study concluded that most patients who followed an unrestricted solid food diet had no increase in complications when compared to patients under dietary restrictions.29 There is a lack of clinical evidence showing benefit for a clear liquid diet during an acute episode of diverticulitis.7
Clinicians should, however, consider patient preference when discussing the management of acute diverticulitis. For example, patients experiencing nausea or anorexia may prefer to follow a clear liquid diet for two to three days, as it provides more comfort/relief.25 They should be advised to resume their normal diet as tolerated.2 Patients who are unable to return to their normal diet within a few days require follow-up and potentially escalation of treatment, e.g. initiation of antibiotics.2, 25
Prescribe appropriate analgesia
Paracetamol is the first-line option for analgesia.2, 21 Anti-spasmodic medicines, e.g. hyoscine butylbromide (Buscopan), may be beneficial for patients experiencing gastrointestinal cramping.2, 21 Non-pharmacological options may also be useful for pain management, e.g. heat packs or relaxation techniques.
International guidelines are unclear on the role of NSAIDs and weak opioids in the management of pain associated with acute diverticulitis.2, 3 In patients with no risk factors for complicated diverticulitis and who can maintain adequate oral intake (food and fluid), a short course, i.e. 48 hours, of NSAIDs could be considered. Strong opioids (e.g. morphine) should be avoided in most situations due to an increased risk of bowel obstruction and perforation, however, immediate-release tramadol may be considered for patients with severe pain not responsive to paracetamol (but not severe enough to require referral to hospital).2, 30
Reconsider the need for oral antibiotics for patients managed in the community
Oral antibiotics are no longer recommended as a first-line treatment for acute diverticulitis in most patients with uncomplicated disease and no co-morbidities.10, 21 ,23 International guidance states that antibiotics should be reserved for patients with sepsis or immunocompromise.10 However, this recommendation is reliant upon having a CT-confirmed diagnosis of diverticulitis. Without direct access to imaging, clinicians will need to use clinical judgement to determine which patients treated in the community may benefit from antibiotics, e.g. those who are at higher risk of complications, taking certain medicines (such as corticosteroids, anticoagulants) or systemically unwell but not yet meeting local criteria for hospital referral.21 See Table 1 for choice of antibiotic regimen.
Also consider antibiotics in patients whose condition has not improved after 48 hours of conservative community management22
Table 1. Antibiotic regimens for adult patients with suspected acute uncomplicated diverticulitis.34
| First-line dual treatment |
| Metronidazole |
400 mg, three times daily, for five or seven days* |
| PLUS, ONE OF EITHER: |
|
Trimethoprim + sulfamethoxazole |
960 mg, twice daily, for five days |
| Amoxicillin |
500 mg, three times daily, for seven days |
| Cefalexin |
500 mg, two to three times daily (maximum 1 – 1.5 g, three to four times daily), for five days |
| Alternative monotherapy: |
| Amoxicillin + clavulanic acid |
Adult: 625 mg, three times daily, for five days |
*Give seven days course of metronidazole if prescribed with amoxicillin
Community management without antibiotics requires appropriate patient follow-up
Patients being managed conservatively in the community with acute uncomplicated diverticulitis should ideally be followed up in 48 hours or earlier depending on their clinical condition.22 Follow-up provides an opportunity to reassess the patient’s symptoms, discuss the reintroduction of dietary fibre if appropriate and reconsider the need for oral antibiotics (or referral to secondary care).
Patients should also be clearly advised of the possible red flag symptoms that would indicate that hospital, rather than community management, is appropriate, such as increasing abdominal pain, ongoing vomiting, persistently elevated fever, inability to eat and per rectum bleeding (see: “Red flag symptoms and signs suggesting complicated diverticulitis”).2, 21
Antibiotics are no longer the cornerstone of management for acute uncomplicated diverticulitis
Insufficient clinical evidence for the use of antibiotics in patients with mild symptoms, combined with wider acceptance of an inflammatory aetiology of acute diverticulitis, drives this change in clinical practice.31 Other concerns include rising antibiotic resistance rates and adverse effects associated with the use of antibiotics.20 A 2022 systematic review, including three randomised controlled trials, evaluated antibiotic treatment in patients with confirmed, uncomplicated acute diverticulitis against no antibiotic treatment; a conclusion regarding the benefit of antibiotic treatment could not be reached.32 Patients who received oral antibiotics did not show statistically significant differences in time to recovery or progression to complicated diverticulitis compared with patients who did not receive oral antibiotics.32 Additionally, a New Zealand-based placebo-controlled randomised controlled trial demonstrated that antibiotics did not reduce hospital stay in patients with CT-proven uncomplicated diverticulitis,33 further supporting a move away from regular antibiotic use.
In patients who have recovered from acute diverticulitis, the goal of ongoing management is to prevent future episodes. Lifestyle modifications to prevent the initial development of diverticulitis in patients with diverticulosis, e.g. reducing red meat intake and increasing dietary fibre, could potentially prevent the development of future episodes (“Managing diverticulosis”).25 International guidelines often recommend that patients with a history of acute diverticulitis adhere to high-fibre diets, however, there is limited evidence to support this.7, 10, 35
Approximately one in five patients who experience acute diverticulitis will have at least one acute recurrence within the next ten years; the more episodes experienced or a previous complicated episode, increases the risk of recurrence.17, 25
Chronic diverticulitis is when patients experience ongoing gastrointestinal symptoms, lasting at least three months despite treatment with oral antibiotics.36, 37 Symptoms often begin to improve during antibiotic treatment but then regress after treatment ceases.36 Management of patients with chronic diverticulitis should be discussed with a gastroenterologist. In rare cases, patients may be considered for a surgical treatment option, e.g. segmental resection of the affected areas of bowel.36, 37
Pharmacological prophylaxis is not recommended10, 25
One area of ongoing research is the use of anti-inflammatory medicines to reduce the risk of recurrent diverticulitis or manage chronic symptoms.10, 36 Both mesalazine (5-aminosalicylic acid), an aminosalicylate anti-inflammatory medicine, and rifaximin*, a broad-spectrum antibiotic with anti-inflammatory properties, have been studied for their use in patients with diverticulitis,6, 36 however, there is a lack of clear evidence of benefit, therefore guidelines do not currently recommended them.10, 36
*Rifaximin is only funded with Special Authority approval for hepatic encephalopathy in New Zealand34
Some patients will require follow-up colonoscopy to exclude colorectal cancer
Colorectal cancer is associated with complicated diverticular disease,38 and in some situations, cancer symptoms may be misattributed to an episode of diverticulitis.2 Appropriate follow-up of patients with diverticulitis is therefore crucial to rule out colorectal cancer, and can be summarised as:
- Patients with complicated, CT-proven diverticulitis require a colonoscopy to rule out cancer
- Patients with uncomplicated, CT-proven diverticulitis do not require a colonoscopy because the risk of cancer is lower, and a CT scan is sufficient in most cases to assess for abnormalities
- The benefits of colonoscopy in patients with suspected, uncomplicated diverticulitis (who have not received a CT scan) are unclear – refer for colonoscopy if other risk factors are present
Patients who have had an episode of complicated diverticulitis or those who meet the criteria for colorectal cancer surveillance should receive a non-urgent referral for a follow-up colonoscopy.3 This should not be performed for at least six weeks after resolution of symptoms due to risks of bowel perforation.25
Previous guidelines have recommended a follow-up colonoscopy for all patients diagnosed with diverticulitis due to the similarities between the symptoms of acute diverticulitis and colorectal cancer.7 However, when compared to the general population, research has shown no difference between the incidence of colorectal cancer following CT-proven acute uncomplicated diverticulitis and the incidence of colorectal cancer in the general population.7 The need for colonoscopy in patients without a CT-proven diagnosis is less clear. Consider if these patients meet other referral criteria for a colonoscopy.
A follow-up colonoscopy is not required for patients with CT-proven acute uncomplicated diverticulitis, who have no red flag symptoms and who do not meet criteria for colorectal cancer surveillance.7





