Published: 12 August 2022
Key practice points:
- Coeliac disease is an immune-mediated condition where exposure to gluten results in inflammation of the small intestine and flattening of the villi, which affects the absorption of important nutrients including iron, folic acid, calcium and fat-soluble vitamins
- Symptoms of coeliac disease are varied and non-specific:
- Classic symptoms related to the gastrointestinal tract include diarrhoea, abdominal distension, discomfort and weight loss; other gastrointestinal symptoms include constipation, nausea and vomiting
- Extra-intestinal symptoms include chronic fatigue and dermatitis herpetiformis. Anaemia, often secondary to iron deficiency, is a frequent finding.
- Children may also present with faltering growth, short stature or pubertal delay
- Some people may be asymptomatic
- If coeliac disease is suspected, advise the patient to continue with a gluten-containing diet and request laboratory testing for coeliac-specific antibodies, i.e. a “coeliac screen”. Consider other relevant laboratory investigations based on suspected nutrient deficiencies, co-morbidities and differential diagnoses.
- The gold standard for the diagnosis of coeliac disease is positive coeliac-specific serology results and histological changes of the small intestinal mucosa on duodenal biopsy, while the patient is consuming gluten; however, biopsy is not always necessary. Following a positive coeliac serology result, refer adults to a gastroenterologist for further assessment.
- Children may be diagnosed with coeliac disease without a duodenal biopsy based on strict criteria, including strongly suggestive laboratory results on two separate blood samples. All children with suspected coeliac disease should be referred to a paediatric gastroenterologist for a formal diagnosis.
- A strict gluten-free diet is the only effective treatment for people with confirmed coeliac disease. Pharmacological management of co-morbidities may be required, e.g. iron deficiency.
- People with untreated coeliac disease have an increased risk of long-term health conditions such as osteoporosis, lymphoproliferative disorders and gastrointestinal malignancy. Risk usually reduces following a strict gluten-free diet.
Coeliac disease is a chronic inflammatory condition of the small intestine that causes flattening of the villi and results in nutrient malabsorption.1 Coeliac disease occurs in genetically susceptible people and involves an autoimmune response to gluten – the major protein found in varieties of wheat, rye and barley.1, 2 It is not an allergy to gluten. A small proportion of people with coeliac disease may also be affected by avenin, the protein found in oats (see: “The role of oats in a gluten-free diet”). Coeliac disease is associated with an increased risk of long-term health problems, e.g. osteoporosis, infertility, lymphoproliferative disorders (e.g. T-cell lymphoma) and gastrointestinal malignancy.1, 3 ,4 However, this risk reduces in people who, after diagnosis, adhere to a strict gluten-free diet (see: “Complications of coeliac disease”).1, 3, 4
The prevalence of coeliac disease has increased over time
The prevalence of coeliac disease is increasing, which may partially reflect increased awareness and testing.2, 5 It is estimated that 1.2% of adults in Australasia have coeliac disease; exact prevalence data are unavailable for children, however, rates are reportedly increasing.6 Worldwide, prevalence data range from 0.6 – 1.9% and risk varies according to several factors:1, 2, 6–9
- Genetics. People with human leukocyte antigen (HLA) haplotypes, HLA-DQ2 or HLA-DQ8, or people with a family history of coeliac disease – particularly first-degree relatives, have the strongest genetic predisposition to developing coeliac disease. People with an autoimmune condition, e.g. type 1 diabetes, autoimmune thyroid or liver disease, trisomy 21, Turner or Williams syndrome and those with IgA deficiency are also at greater risk of coeliac disease than the general population.1, 9, 10
- Ethnicity and geographic location. Coeliac disease occurs in people living in any geographic area and of any ethnicity, however, it predominantly affects European populations.3, 4, 11 In New Zealand, children of Indian ethnicity are reportedly disproportionately affected.
- Age. A diagnosis of coeliac disease can be made at any age; however, most people are diagnosed as adults, often between the ages of 40 and 45 years.1, 7, 12
- Sex. Studies report that a higher proportion of females have coeliac disease than males (approximately 1.3 to 1.5 females are affected for every one male).1, 7
N.B. There is no evidence that breastfeeding duration or timing of gluten introduction influences the development of coeliac disease.1
Coeliac disease is often under-recognised because of the wide range and non-specific nature of symptoms with which people present.10, 13
The ‘classical’ presentation of coeliac disease reflects its effect on the small intestine causing gastrointestinal symptoms, e.g. diarrhoea, abdominal distension and discomfort, weight loss or in children, failure to gain weight (Figure 1).1 Symptoms of coeliac disease may be difficult to distinguish from those found in irritable bowel syndrome or inflammatory bowel disease.1 People may also present with other gastrointestinal symptoms such as constipation, nausea and vomiting, or extra-intestinal manifestations, e.g. chronic fatigue, dermatitis herpetiformis, anaemia secondary to iron deficiency, or they can be asymptomatic.1, 14 Children may display additional symptoms and signs, e.g. faltering growth, pubertal delay, emotional distress/changes in mood.1, 5
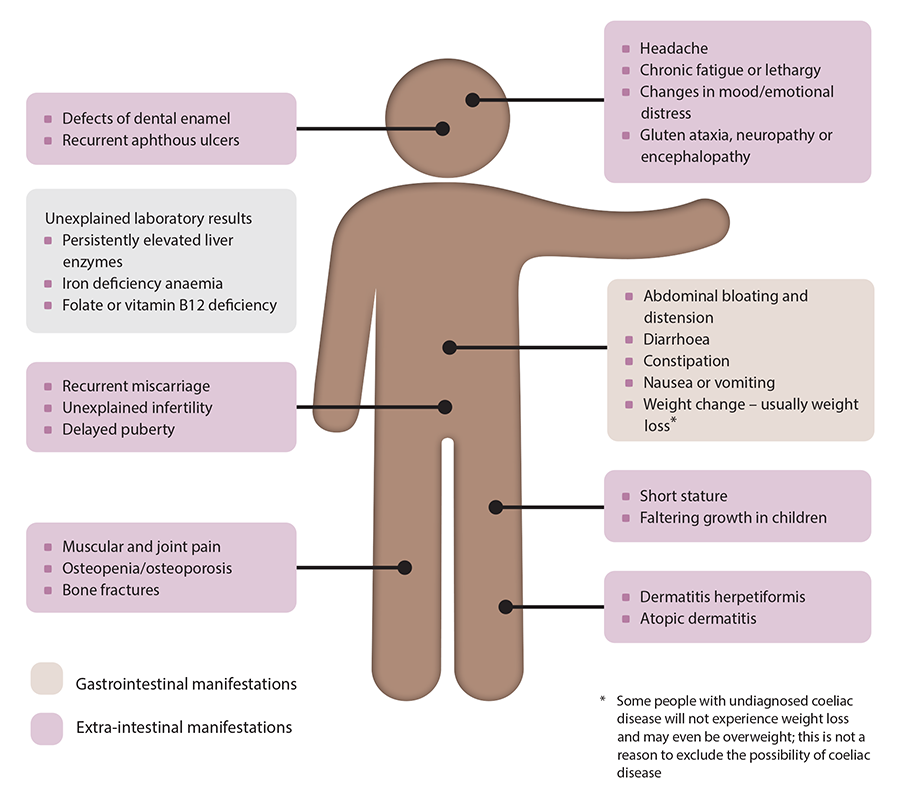
Figure 1. Coeliac disease – symptoms, signs and associated conditions.1, 9, 10, 14
N.B. Not all people with coeliac disease will display these symptoms and signs, some people can also be asymptomatic.
In patients with suspected coeliac disease:
- Determine the frequency, severity and duration of symptoms
- Consider other factors or conditions that could cause the reported symptoms, e.g. medicines, stress, other dietary triggers or intolerances, irritable bowel syndrome or inflammatory bowel disease. N.B. Some of these may co-exist with coeliac disease.
- Assess for risk factors, e.g. family history, autoimmune disease, underlying genetic condition
- Perform a physical examination
- Measure height and weight and calculate BMI as a baseline. For children, plot growth on a centile chart, preferably with longitudinal data.
- Palpate the abdomen for any tenderness, masses or other abnormalities
- Check skin for any rashes
- Check mouth for ulcers, dental enamel defects, angular stomatitis
- Assess for any signs of anaemia
Practice point: Exacerbation of symptoms following gluten consumption should not be used as a diagnostic measure in the absence of other investigations as it has a very low predictive value for coeliac disease.1 Conversely, dietary tolerance of gluten does not rule out coeliac disease.
Coeliac disease is an immune-mediated response to gluten; therefore, antibody testing has an important role in the diagnosis. The reliability of these tests has improved over time.1 Serum testing for coeliac-specific antibodies is indicated for all patients in whom coeliac disease is suspected, including those who are asymptomatic but at high risk of coeliac disease, e.g. due to family history, a genetic condition or other associated condition (e.g. unexplained infertility, autoimmune thyroid disease, type 1 diabetes).1, 9
Which laboratory tests to request
Coeliac disease is associated with elevated tissue transglutaminase (tTG), endomysial (EMA), deamidated gliadin peptide (DGP) and anti-gliadin (AGA)* antibodies.1
Request a “coeliac screen” on the laboratory form and advise the patient to continue with their usual diet, i.e. containing gluten (see: “Patients should be consuming gluten in the lead up to testing for coeliac disease”).
The specific antibody tests performed by the laboratory when a request is made for a “coeliac screen” varies. Generally, the laboratory will begin by testing serum anti-tTG and total IgA. If anti-tTG is positive (and IgA levels are sufficient), EMA-IgA is usually then tested automatically. Testing the total IgA level is necessary as up to 2.6% of people with coeliac disease have IgA deficiency that can give a misleadingly low anti-tTG result.4, 9 If IgA levels are deficient, DGP-IgG is usually then tested automatically.9 DGP is not as reliable as tTG in people with normal IgA titres, therefore, use outside of IgA deficiency is not recommended.4 If results are uncertain and clinical suspicion of coeliac disease remains for a patient with IgA deficiency, discuss with or refer to a gastroenterologist.
*N.B. AGA antibodies are no longer used in the testing panel for coeliac disease due to low specificity.12
Request additional laboratory tests, as indicated
Additional laboratory tests may be requested to investigate differential diagnoses, e.g. inflammatory bowel disease, or associated conditions, e.g. autoimmune hepatitis or thyroid disease. Also consider testing for and treating any nutritional deficiencies or abnormal biochemistries as the inflammatory changes associated with coeliac disease can disrupt intestinal absorption.1
Laboratory tests may include:1
- Full blood count
- Ferritin
- LFTs
- TSH (as higher risk of autoimmune thyroid disease)
- Folate
- Vitamin B12
- Calcium and phosphate
- CRP (as indicated)
- Faecal calprotectin (if inflammatory bowel disease suspected)
Patients should be consuming gluten in the lead up to testing for coeliac disease
A negative result for coeliac-specific antibodies does not exclude coeliac disease if the patient has reduced or removed gluten from their diet prior to testing. If the patient is not consuming gluten, a gluten challenge is required (see below).4 If the patient is unable to tolerate a gluten challenge, HLA typing (genetic testing) may be considered to exclude coeliac disease if the test is negative; however, this test is usually reserved for use by a gastroenterologist for when a diagnosis of coeliac disease is uncertain (see: “HLA typing may be requested by a gastroenterologist to exclude coeliac disease in selected patients”).1
Gluten challenge
The quantity of gluten and the duration of the challenge required to produce the histological and serological evidence characteristic of coeliac disease varies.12
 In general, a gluten challenge for adults is ≥ 5 g of gluten per day* for three to eight weeks.1 If not tolerated, reduce the amount of gluten being consumed (also see Best Practice Tip) or if significant symptoms occur, the challenge can be stopped after a minimum of two weeks.7
In general, a gluten challenge for adults is ≥ 5 g of gluten per day* for three to eight weeks.1 If not tolerated, reduce the amount of gluten being consumed (also see Best Practice Tip) or if significant symptoms occur, the challenge can be stopped after a minimum of two weeks.7
*For example, equivalent to a minimum of two to four slices of wheat bread; or two to four weetbix; or half to one cup of cooked pasta per day12
A gluten challenge may be appropriate for children under medical supervision from a paediatric gastroenterologist, however, it should be avoided in those aged under five years and during pubertal growth spurts.1
Best Practice Tip. If the patient is concerned about the tolerability of gastrointestinal symptoms during a gluten challenge, recommend gluten-containing products that contain fewer fermentable sugars, e.g. spelt flour-based breads, or consuming small amounts of gluten across multiple meals rather than all at once.12
A duodenal biopsy may no longer be essential for the diagnosis of coeliac disease
Following positive coeliac-specific serology results, advise the patient to continue eating a gluten-containing diet and organise referral to a gastroenterologist for further assessment (Figure 2).1 In the past, duodenal biopsy was regarded as the gold standard investigation for the diagnosis of coeliac disease, but due to advancements in the reliability and accuracy of serological testing over time, some gastroenterologists now consider strongly suggestive serology results to be sufficient for diagnosis, particularly in children (see: “A diagnosis of coeliac disease can be made in some children without a duodenal biopsy”) and now increasingly in selected adults, e.g. those aged under 50 years without red flag symptoms and signs.15 Some gastroenterologists also diagnose coeliac disease without duodenal biopsy in people with confirmed dermatitis herpetiformis.
In some cases, genetic testing may be considered if the diagnosis of coeliac disease is uncertain (see: “HLA typing may be requested by a gastroenterologist to exclude coeliac disease in selected patients”).1

Figure 2. Diagnostic overview of coeliac disease in adults.12
A duodenal biopsy can still be useful for some patients
 Histological changes characteristic of coeliac disease include flattening of the villi, crypt hyperplasia, a thickened basement membrane, raised intraepithelial lymphocytes and inflammatory cell infiltrate of the lamina propria.4, 5
Histological changes characteristic of coeliac disease include flattening of the villi, crypt hyperplasia, a thickened basement membrane, raised intraepithelial lymphocytes and inflammatory cell infiltrate of the lamina propria.4, 5
A biopsy may still be recommended for a patient for the following reasons:14–16
- For diagnostic certainty as:
- Elevated anti-tTG titres could represent a false-positive result
- Some patients find reassurance in having a histologically confirmed diagnosis
- Adherence to a gluten-free diet is a lifelong commitment and usually has a significant effect on the patient’s quality of life, including financial implications
- To assess mucosal damage. Serological testing may not always correlate with mucosal damage, and it is important to accurately determine the degree of inflammation and villous changes in the small intestine as well as excluding other small intestinal diseases.
- Due to implications for future medical care, e.g. increased risk of malignancy
- For Special Authority approval of partly funded gluten-free products.* N.B. A biopsy is not required for Special Authority approval in children where a biopsy-free diagnosis has been made by a paediatric gastroenterologist.
*Funding of these products is no longer actively managed by PHARMAC (i.e. no access, product or funding changes will occur), therefore, the range of funded items will decrease over time17
Although rare, false negative and false positive results can still occur with duodenal biopsy, e.g. if the sample is taken in an area without damage, or if medicine-related adverse effects or an infection produces histological changes consistent with those of coeliac disease.14–16
If clinical suspicion for coeliac disease remains despite normal histological findings, consider re-referral to a gastroenterologist for further assessment if this has not already been organised.1
A diagnosis of coeliac disease can be made in some children without a duodenal biopsy
All children with suspected coeliac disease should be referred to a paediatric gastroenterologist. Check local HealthPathways for specific referral criteria. A diagnosis of coeliac disease can be made by a paediatric gastroenterologist in some children without the need for a duodenal biopsy (Figure 3) based on the following criteria from the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Guidelines for Diagnosing Coeliac Disease (2020):13
- Anti-tTG IgA ≥ 10 × the upper limit of normal (ULN); plus
- Confirmation with EMA-IgA (or DGP-IgG if IgA deficiency) and a positive anti-tTG result in a second serum sample. N.B. The tTG on the second sample does not need to be ≥ 10 × ULN.
The ESPGHAN criteria for a biopsy-free diagnosis of coeliac disease in children has been validated in a New Zealand population.18 Regions across New Zealand are working on implementing these guidelines locally. The role of a non-biopsy diagnosis for adults with suspected coeliac disease is also evolving based on this approach.

Figure 3. Diagnostic overview of coeliac disease in children.10, 13
HLA typing may be requested by a gastroenterologist to exclude coeliac disease in selected patients
Susceptibility genes, HLA-DQ2 and HLA-DQ8, are present in almost all people with coeliac disease.1 If the patient tests negative for HLA-DQ2/8, coeliac disease is very unlikely.1 A positive HLA typing result indicates that coeliac disease is possible, however, it does not provide diagnostic certainty as these haplotypes are also present in up to 40% of the general population who do not have coeliac disease.4, 13 The positive HLA test must be followed up with coeliac-specific antibody testing (if not already performed) with the patient still consuming gluten, and managed according to the results.19
HLA typing has a limited role in the diagnosis of coeliac disease because of its high negative predictive value, however, it may be requested by a gastroenterologist to exclude coeliac disease (if the result is negative) if the diagnosis is uncertain, e.g. discrepant findings in a patient who is symptomatic.1, 12
Expert tip: “HLA testing to exclude coeliac disease is not helpful in people with type 1 diabetes as the HLA associations are the same, so they will invariably be positive by virtue of having type 1 diabetes.”
First-degree relatives should be checked for coeliac disease
Following a confirmed diagnosis of coeliac disease, advise the patient that their first-degree relatives should also be checked for coeliac disease using serology.1
- Testing young children with a family history of coeliac disease can begin after age two years, or earlier if they are symptomatic, and may be appropriate every two to three years due to the risk of untreated coeliac disease on growth and bone health12, 20
- For asymptomatic adults with a family history of coeliac disease, the interval between repeat antibody testing is unclear; it may be appropriate to repeat coeliac serology every few years, or alternatively, only repeat if symptoms suggestive of coeliac disease develop12, 20
Expert Advice: “In theory HLA typing may be used to check first-degree relatives for coeliac disease as if the result is negative, you would not need to test again, however, in practice the test is rarely helpful and often confuses the issue given the large proportion of New Zealand Europeans who test positive for HLA-DQ2/8. Plus, a positive result only tells you whether someone is susceptible to coeliac disease, not whether they have coeliac disease at the time of testing or whether they will develop it in the future. We advise checking first-degree relatives with serology, but some clinicians do test HLA if the relative also has additional risk factors for coeliac disease which mean they would require lifelong testing, e.g. trisomy 21. These people can then avoid the need for lifelong serology testing for coeliac disease if the HLA result is negative.”
What to do after negative coeliac-specific serology results
If the results of coeliac-specific antibody testing are negative, but the patient remains symptomatic, reconsider differential diagnoses, e.g. irritable bowel syndrome or inflammatory bowel disease. Alternatively, discuss with or refer to a gastroenterologist for further assessment. Check local HealthPathways for specific referral criteria.
Adherence to a gluten-free diet, i.e. exclusion of all foods containing wheat, rye and barley, is key for the management of coeliac disease.1 Following a confirmed diagnosis, offer referral to a dietitian if this has not already been organised by the gastroenterologist.1 Also recommend that patients or their parents/caregivers join local support groups and register with Coeliac New Zealand; a range of resources, advice and assistance is available on their website: coeliac.org.nz
Educate, support and encourage patients transitioning to a gluten-free diet
Patients should be provided with ongoing support, education and reassurance when changing to and sustaining a gluten-free diet.1 Adherence to a strict gluten-free diet is challenging, even in ideal circumstances and can be complicated by family and professional commitments as well as fears of social isolation or being different.1 Food is also often a significant aspect of family and cultural traditions.
With the help of Coeliac New Zealand, peer support groups, a dietitian and their general practice team, people with coeliac disease should be educated and advised on:
 How to eat a balanced, varied, gluten-free diet
How to eat a balanced, varied, gluten-free diet
Strict adherence to a gluten-free diet generally corrects any initial nutritional deficiencies that were due to malabsorption. However, some gluten-free diets can be low in nutrients including, fibre, calcium, vitamin D, vitamin B12, folate or iron; advise patients to eat a balanced and varied diet (e.g. high-fibre with whole-grain rice, corn, potatoes and vegetables) with appropriate substitutions, and consider supplementation as required.1, 3
 The role of oats in a gluten-free diet
The role of oats in a gluten-free diet
Oats contain high levels of some vitamins, minerals and fibre which can improve the nutritional adequacy and variety of a gluten-free diet. Some people with coeliac disease, however, do not tolerate oats (possibly due to avenin toxicity/sensitivity), and there can also be cross contamination with gluten during production; there is no reliable source of gluten-free oats available in New Zealand.19 For these reasons, Coeliac New Zealand does not recommend the consumption of oats for people with coeliac disease. However, this is still somewhat of a “grey area”, for example, some paediatric gastroenterologists may recommend that oats can be re-introduced for children under medical supervision after tTG has normalised, with monitoring of serology. Flaked buckwheat, quinoa or other gluten-free grains may be suitable alternatives in those who cannot tolerate oats.
 Product availability
Product availability
Gluten-free foods are usually widely available in supermarkets and via gluten-free food supplier websites. A small selection of gluten-free products, e.g. flour, baking mix, bread mix and pasta, are available on prescription (partly funded with Special Authority approval*).17 N.B. The funding of these products is no longer actively managed by PHARMAC (i.e. no access, product or funding changes will occur), therefore, the range of funded items will decrease over time.17 Prescription gluten-free products may not be less expensive than supermarket equivalents when consultation and other prescription-associated fees are considered. Some people with coeliac disease (or caregivers) may also be eligible to receive a disability allowance; for further information, see: coeliac.org.nz/winz-info
*Partly funded for patients with a biopsy-proven diagnosis of coeliac disease (or for children with a biopsy-free diagnosis of coeliac disease according to ESPGHAN criteria) or if the patient has dermatitis herpetiformis. Prescription gluten-free products are only available at hospital pharmacies (home delivery may be available in some areas).
 How to interpret food labels
How to interpret food labels
It can be difficult for people newly diagnosed with coeliac disease to establish which food products are gluten-free as many contain ingredients made from gluten-containing grains that may not be immediately obvious. The Australia New Zealand Food Standards Code established under The Food Standards Australia New Zealand (FSANZ) Act 1991 requires that food labels must declare if the product contains an ingredient derived from a cereal containing gluten, e.g. wheat, rye, barley (and malt derived from barley), spelt and oats.21 A full list of gluten-containing grains is available from: coeliac.org.nz/common-sources-of-gluten
For a product to be classified as gluten-free in New Zealand it must contain no detectable gluten, i.e. less than three parts per million of gluten.19, 21 Coeliac New Zealand licences the use of the “Crossed Grain” logo to manufacturers which certifies that products are suitable for a gluten-free diet.19
Foods labelled as “low gluten” or “contains traces of gluten” are not usually suitable for a person with coeliac disease.
Many product labels state “may contain gluten” but are gluten-free by ingredient. Although these foods may not necessarily produce symptoms related to gluten exposure, there is a risk that intestinal damage can occur, especially if eaten frequently or in large quantities. This labelling is not a FSANZ requirement, and usually means that the manufacturer cannot guarantee that the product does not contain gluten, e.g. there may be a risk of cross-contamination because it was processed in a manufacturing plant that also produces gluten-containing products. In practice, many people with coeliac disease choose to consume “may contain” foods so that their diet is not too restrictive; encourage these patients to be vigilant for any symptoms, and have a low threshold for serology testing if symptoms are reported.
 Cross contamination and hidden sources of gluten
Cross contamination and hidden sources of gluten
Common examples include foods containing additives or thickeners derived from wheat, some confectionery, desserts, coated oven fries, processed meats, sauces (including some soy sauces), dressings and soups. Some medicines may contain gluten as an excipient but usually only in small amounts; continued use, however, can cause ongoing mucosal damage and symptoms in some people with coeliac disease. Before prescribing a medicine to a patient with coeliac disease, check the excipient list for gluten. Certain children’s play materials may also contain gluten, e.g. synthetic modelling doughs and plasticines. Involvement of caregivers and teachers can help to prevent “cheating” or experimentation with the diet, sharing lunchboxes at school, parties or other accidental exposure to gluten.
Smartphone apps are available to support people living gluten-free with coeliac disease. These apps range from enabling food and symptom tracking, to providing gluten-free recipes and food substitutions. For further information, see: www.healthnavigator.org.nz/apps/c/coeliac-disease-apps
A gluten-free diet improves symptoms and long-term health for people with coeliac disease
A strict lifelong gluten-free diet usually resolves the majority of symptoms associated with coeliac disease, normalises coeliac-specific antibody titres and heals the small intestinal mucosa.12
After 6 – 12 months of adherence to a gluten-free diet, approximately 80% of people with coeliac disease will test negative for coeliac antibodies or show a considerable reduction in tTG titre, and by five years of adherence, this increases to more than 90%.1 The rate of histological improvement is comparatively slower; studies show that mucosal healing can take up to two years for children and up to eight years for adults.1, 14
Eliminating gluten from the diet also lowers the risk of complications associated with coeliac disease, e.g. osteoporosis, infertility (see: “Complications of coeliac disease”).1, 3, 4
It is estimated that fewer than one in 100 people with coeliac disease will not respond to a strict gluten-free diet and require further gastroenterology review (see: “Consider the possibility of non-responsive or refractory coeliac disease”).4
Complications of coeliac disease
Coeliac disease is associated with an increased risk of long-term health problems (see below), however, those who adhere to a strict gluten-free diet, are generally at lower risk than people with untreated coeliac disease.1, 3, 4 People with coeliac disease also have up to ten times greater risk of having or developing another autoimmune condition, e.g. type 1 diabetes, autoimmune thyroid disease, autoimmune hepatitis, primary biliary cholangitis, Sjögren’s syndrome, systemic lupus erythematosus and systemic sclerosis, compared to those without coeliac disease.5, 10, 22
Complications of coeliac disease can include:
- Osteoporosis. Bone densitometry scans can be requested on a case-by-case basis, e.g. aged > 55 years or with additional risk factors for osteoporosis.23 Encourage adequate intake of calcium and vitamin D. Bone mineral density increases during the first year of a gluten-free diet as nutrient absorption improves.1
- Dermatitis herpetiformis occurs in up to 25% of people with coeliac disease and is characterised by an itchy, blistering skin eruption predominantly affecting the knees, elbows, buttocks and back.24 A strict gluten-free diet may adequately control the dermatitis, although specialist treatment with oral dapsone may be required for some patients.24
- Malignancy, e.g. T-cell lymphoma and gastrointestinal malignancies, particularly small intestinal carcinoma.1, 14 Refractory coeliac disease can signify the development of T-cell lymphoma. Strict adherence to a gluten-free diet may reduce the incidence of gastrointestinal-related malignancies, however, evidence is more limited for others, e.g. lymphoma.1
- Temporary lactose intolerance caused by reduced lactase production due to changes in intestinal villi.25 Lactose should be gradually re-introduced to avoid unnecessary dietary restrictions and to ensure adequate calcium intake.25 For further information on lactose intolerance, see: bpac.org.nz/2021/lactose-intolerance.aspx
- Obstetric-related, e.g. reduced fertility, miscarriage and pre-term birth.26 Nutrient malabsorption and elevated serum anti-tTG and anti-thyroid antibodies may contribute to the increased risk of these complications, however, evidence is unclear.26
- Cardiovascular-related, e.g. atherosclerosis, cardiomyopathy, thrombosis, ischaemic heart disease, stroke and arrythmia.27 Chronic inflammation, nutritional deficiencies and duodenal mucosal changes are all thought to be contributory factors.27
- Hyposplenism, rare; suggested by abnormalities on the blood count and blood film, but associated with an increased risk of infection1
- Ulcerative jejunitis, rare; usually associated with refractory coeliac disease1
Follow-up is typically more frequent in the first year following a diagnosis of coeliac disease, e.g. ideally every three to six months, while the patient is becoming established on a gluten-free diet.1, 28 Initially, this may be with the gastroenterologist, but for most patients, ongoing follow-up occurs in primary care. Once the patient is successfully established on a gluten-free diet and symptoms have resolved, reviews can be conducted annually.1 People with coeliac disease should also be reviewed during pregnancy.
Suggested approach to follow-up appointments:1, 9
- Check BMI or monitor growth in children
- Ask about any persistent or new symptoms. If indicated, perform a physical examination, e.g. abdominal palpation for any tenderness, mass or another abnormality.
- Check how the patient is coping with the gluten-free diet. If there are concerns about inadvertent exposure, repeat coeliac serology and consider re-engagement with a dietitian. Coeliac serology should be repeated after 12 months of a gluten-free diet regardless of symptoms.
- Repeat laboratory tests, e.g. ferritin, folate and vitamin B12, particularly if there were abnormal biochemistries at diagnosis and if symptoms suggest, check for other laboratory evidence of autoimmune conditions, e.g. with LFTs, TSH. If nutritional deficiencies have not normalised or improved after one year of a gluten-free diet (or earlier as appropriate, e.g. older age), consider supplementation.
- Discuss ways to optimise bone health. Bone densitometry scans can be requested on a case-by-case basis, e.g. aged > 55 years or with additional risk factors for osteoporosis.23
Vaccination: People with coeliac disease should be vaccinated against pneumococcus (not funded) due to the increased risk of hyposplenism; the benefit of other vaccinations such as Haemophilus influenzae type b (not funded), meningococcus (not funded) and annual influenza vaccine (funded) to people with coeliac disease are less clear, although may be considered.1, 28
 Consider the possibility of non-responsive or refractory coeliac disease in patients who have had an inadequate response to a gluten-free diet after 12 months, i.e. persistent symptoms, if other diagnoses have been excluded.1, 9 Refer the patient to a gastroenterologist for further assessment. Check local HealthPathways for specific referral advice.
Consider the possibility of non-responsive or refractory coeliac disease in patients who have had an inadequate response to a gluten-free diet after 12 months, i.e. persistent symptoms, if other diagnoses have been excluded.1, 9 Refer the patient to a gastroenterologist for further assessment. Check local HealthPathways for specific referral advice.
“Tighten up your glute-n facts!” with a podcast from The Curbsiders. Dr Amy Oxtentenko discusses coeliac disease from an American perspective, covering the recognition of classical and non-classical features of coeliac disease, diagnostic testing, management and follow up. Listen to it here: thecurbsiders.com/podcast/300 (skip ahead to ‘12.57’ for the main content). Don’t have time to listen to the whole podcast? Click here for a quick summary.
It’s a steep learning curve: A Patient Perspective
After diagnosis, there is some relief in knowing that you have an illness that is treatable (it wasn’t just the irritable bowel I’d assumed) but strangely there is also a type of grieving process or sadness - that you can never eat certain foods again. It highlights the complicated relationships that we all have with food in our lives. It’s been a huge lifestyle change and initially quite hard to alter long-term habits, given I was diagnosed later in life, but it’s good to find that many of my symptoms have resolved with a gluten-free diet.
Food is such an important part of people’s family traditions – the meals and recipes that are special, that have meaning to your family, might no longer be achievable. I’ve had to rethink some of our favourites and my first gluten-free Christmas was a little different! Many recipes I can adapt, some as yet I can’t.
There is more thought needed when planning meals and more cost involved when grocery shopping. I spend quite a while in the supermarket reading labels (I’m sure this will get easier) and I find that I have go to several different supermarkets because the range of gluten-free options varies. I still need to include gluten-containing foods for the rest of the family, so it increases the grocery budget by doubling up on items and because many gluten-free products are more expensive. By default, the rest of the family become a bit gluten-free too. I can already see that you could easily get into a rut with your food, especially while you are adapting to a gluten-free diet. There seems to be less variety, so you eat the same safe things over and over – like rice crackers and tuna! I have to keep reminding myself that many foods are naturally gluten-free – fruit, vegetables, unprocessed meat, fish, rice, eggs, etc.
Coeliac disease is not a “private” illness, and like people with type 1 diabetes especially, it’s one that you have to think about several times a day - basically every time you eat. It’s always there. If I go to an event, eat out, want takeaways, visit someone or get invited to someone’s house, I have to say that I am gluten-free. I admit there is some mild anxiety about what I will eat, or even if there will be anything I can eat. I’ve taken to having “safe” snacks in my bag all the time, just in case! You also have to have trust in the people who prepare food for you and because gluten is hidden in so many foods it’s easy to get caught out. In restaurants I’ve found it’s important to say that you have coeliac disease, not just that you are gluten-free. I’ve had the odd accidental slip-up and been “glutened” which gave me a recurrence of some of my previous symptoms – it’s a steep learning curve.
Looking at the big picture, it’s far from the worst thing that could have happened, but sometimes it’s just a pain. It’s altered the way I think about coeliac disease at work… I can empathise so much better now and my requests for serology for patients have probably gone through the roof!
Thank you to our very own “Dr Sharyn” for sharing her story with us, which no doubt many people with coeliac disease can relate to. Dr Sharyn Willis is a general practitioner and Senior Medical Advisor with bpacnz. She was diagnosed with coeliac disease in December, 2021.
 There is a
B-QuiCK summary available for this topic
There is a
B-QuiCK summary available for this topic





