For many decades pregnancy categories have been used to indicate the safety of prescribing medicines to pregnant or
breast-feeding women. The New Zealand Formulary used a pregnancy category system from the Therapeutic Goods Administration
(TGA) Australia, based on A, B, C, D or X classifications, and breast-feeding information from the Therapeutic Guidelines
Limited (TGL), Australia.1, 2
The system of using pregnancy categories has several limitations, including that:1, 3
- It does not provide information about risks across different trimesters of pregnancy
- Medicines with a wide range of associated risks could be included in the same category
- The categories imply a grading system, which could lead to prescribing based on the risk category rather than an understanding
of the evidence of risks and benefits for a particular patient
- The single-letter classification system does not provide enough information to support informed decision making by
prescribers and patients
As a result of these limitations, there is an international movement away from using pregnancy safety categories, and
instead providing descriptions of the underlying evidence, degree of severity, timing of effects on the developing fetus
and also indicating areas where there is a lack of evidence.
From 1 October, 2018, the NZF will include advice on the safety of medicines in pregnancy and breast-feeding from Drugs
in Pregnancy and Lactation (used by permission from Wolters Kluwer Health).4 This information will be available in an expandable section in a medicine monograph.
For example, the current information regarding the safety of prescribing metronidazole in women who are pregnant is classified as “B2”:

From 1 October, 2018, the pregnancy information for systemic use of metronidazole will show two headers:
“Human Data Suggest Low Risk” and “Pregnancy Summary”:

Clicking on the arrow icon
 to the right of “Human Data Suggest Low Risk” will open a description of this risk category:
to the right of “Human Data Suggest Low Risk” will open a description of this risk category:

Clicking on the arrow icon to the right of “Pregnancy Summary”  will open a brief overview of the evidence
regarding the safety of using metronidazole during pregnancy, including available information on risks during different trimesters:
will open a brief overview of the evidence
regarding the safety of using metronidazole during pregnancy, including available information on risks during different trimesters:

As another example, the current information regarding the safety of prescribing paracetamol in women who are pregnant or
breast-feeding is “A”; not known to be harmful, with a compatible rating for breast-feeding:

From 1 October, 2018, the updated pregnancy information will show “Human Data Suggest Low Risk” and “Pregnancy Summary” and the
breast-feeding information will show “Compatible”. Clicking on the arrow icons next to any of these headings will reveal further information:



The NZF or NZFC may provide additional advice
For some medicines additional information will be included with a “Further pregnancy advice” or “Further breast-feeding advice” header.
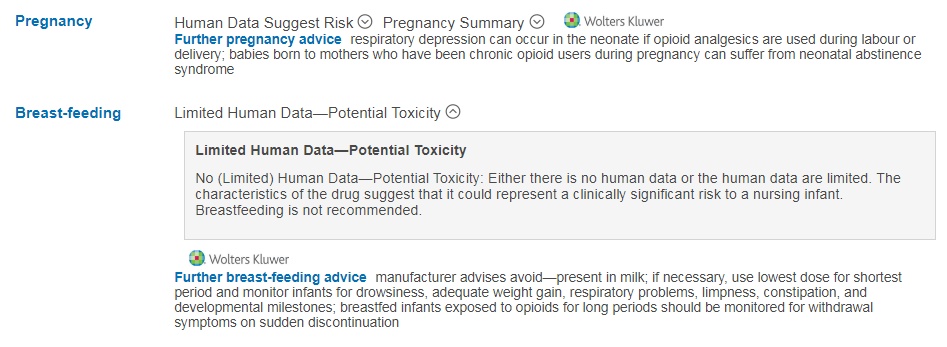
For example, the prescribing recommendations on the use of morphine or allopurinol during breast-feeding will show
“Limited Human Data – Potential Toxicity” with additional advice below under the “Further breast-feeding advice” headers:
Advice for allopurinol use in women who are breast-feeding:

Advice for morphine use in women who are breast-feeding:

Clicking on the “Limited Human Data – Potential Toxicity” header will open a description of this risk category:
Alternative advice will be included if medicine information is not available
For some medicines the NZF or NZFC will provide alternative advice if information regarding its safety in
pregnancy or breast-feeding is not available in Drugs in Pregnancy and Lactation.
Medicine monographs will now also include a “Contraception and conception” section, which will contain information about
any precautionary measures for women or men of childbearing age due to potential teratogenicity, including:
- Recommendations on the type of contraception
- Potential interactions with contraceptives
- Duration of continuing with contraception after a medicine has been stopped






