|
Fluorouracil cream 5%
5-flourouracil, 5-FU |
Imiquimod cream 5% |
Subsidised product |
20 g tube (50 mg/g) |
12 × sachets (12.5 mg/250 mg) |
Mode of Action |
Incorporates into RNA, in turn inhibiting DNA replication and destroying cancerous cells |
An immune response modifier, with anti-viral and anti-tumour action secondary to local cytokine induction
in the skin |
Method of action |
Application is likely to cause erythema, then scaling, tenderness, erosion, ulceration and necrosis. Re-epithelialisation
of the skin then occurs.
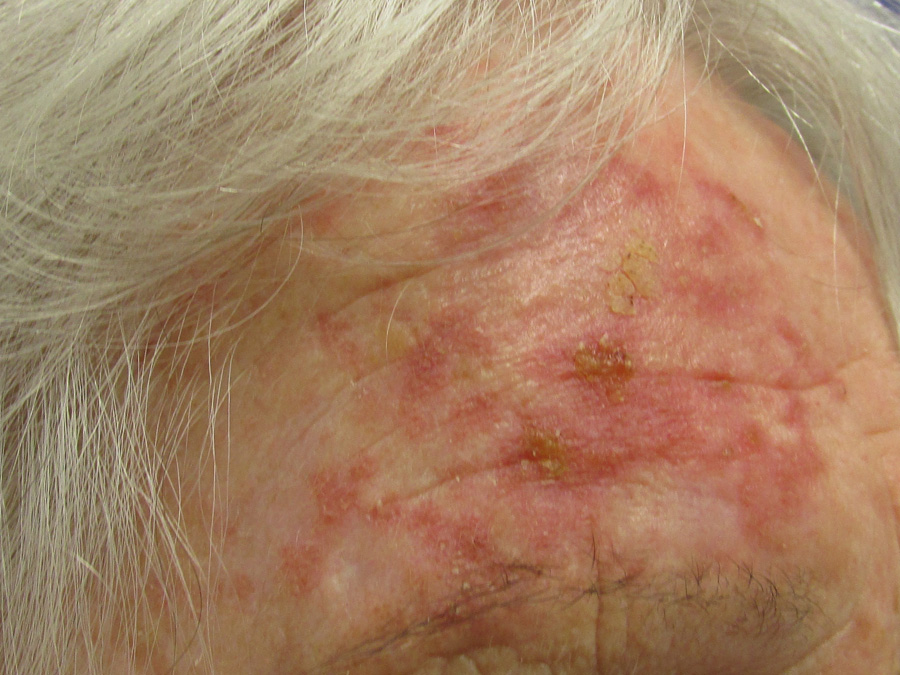
Above: Mild fluorouracil reaction
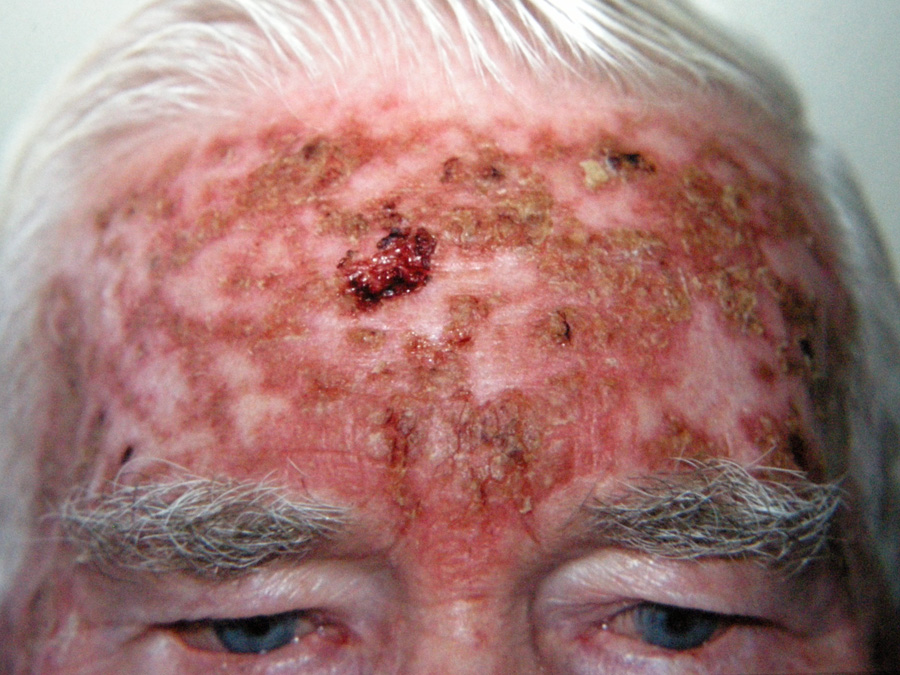
Above: Severe fluorouracil reaction
For further information and images of expected and extreme reactions to fluorouracil treatment,
see: www.dermnetnz.org/topics/5-fluorouracil-cream/ |
Application is likely to cause erythema, erosion, excoriation/flaking, oedema and itching of affected skin.
Painful erosions can occur on mucus membranes.
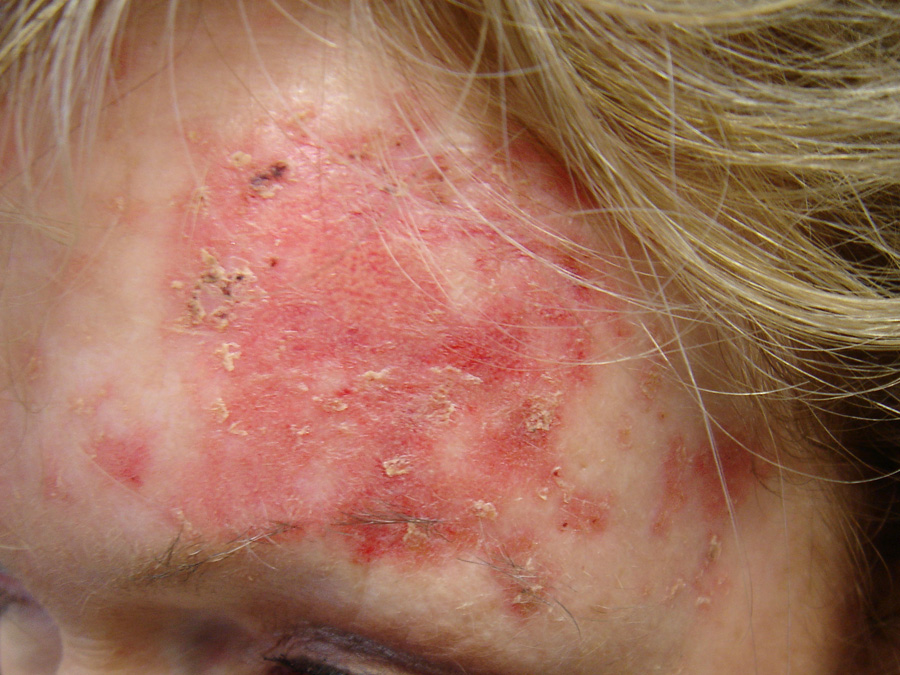
Above: Mild imiquimod reaction
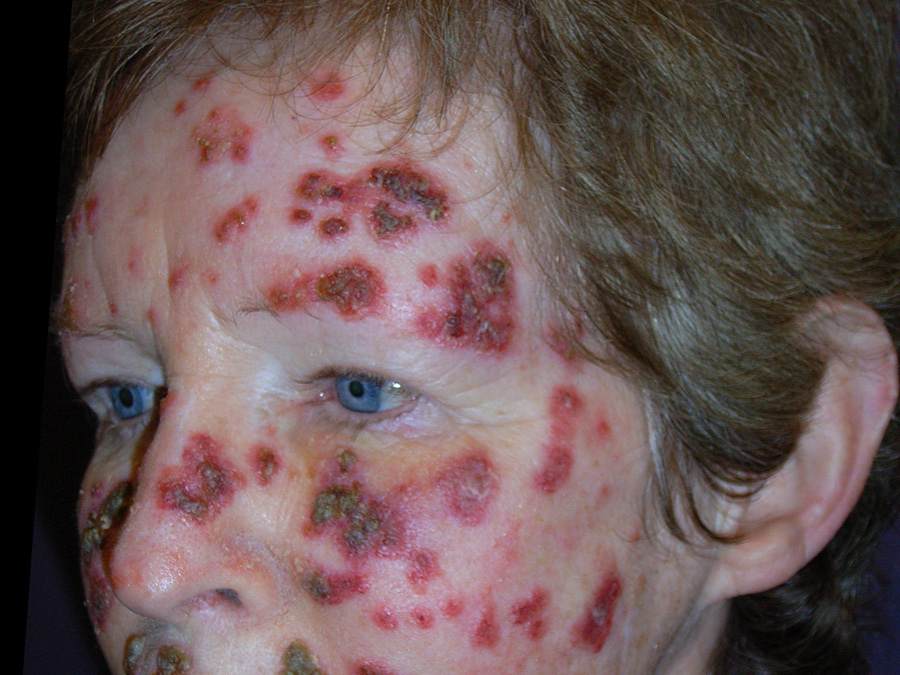
Above: Severe imiquimod reaction
For further information and images of expected and extreme reactions to imiquimod treatment, see:
www.dermnetnz.org/topics/imiquimod/ |
Other adverse effects |
Some patients may experience an intense reaction, including pain, burning, pruritus, rash, crusting, allergic
contact dermatitis, hyperpigmentation, scarring, inflammation and photosensitivity
May result in secondary bacterial infection (via skin erosions)
May cause systemic symptoms including fever, nausea, diarrhoea, headache and mouth ulcers
May cause leukocytosis or leukopenia
Rarely associated with erythema multiforme |
May cause localised hypopigmentation and hyperpigmentation, which has been reported to be permanent in some
patients
May cause flu-like symptoms, e.g. fever, nausea, diarrhoea, headache and myalgia (symptoms are usually tolerated
with paracetamol)
May cause reductions in haemoglobin, white blood cell count, absolute neutrophils and platelets
Rarely associated with erythema multiforme, Stevens-Johnson syndrome and cutaneous lupus erythematosus-like
effect |
Contraindications and cautions for use |
Avoid use in pregnant or breastfeeding women
Avoid contact with eyes or mucus membranes, unaffected skin, broken skin or open wounds
Use cautiously in the perioral area or nasolabial fold, and on lesions below the knees as long-term ulceration
may result from poor healing
Occlusion increases adverse effects
Avoid prolonged exposure to UV light as this will intensify the reaction to fluorouracil |
Use with caution in pregnant or breastfeeding women
Use with caution in patients who are immunosupressed, have autoimmune disease or haematological abnormalities
Avoid contact with eyes or mucous membranes, unaffected skin, broken skin or open wounds
Not photosensitising, but prolonged exposure to UV light should still be avoided |
Pre-treatment |
When treating a hyperkeratotic lesion, pre-treatment with cryotherapy and/or a keratolytic such
as 2% salicylic acid, urea cream or glycolic acid lotion will reduce scaling and therefore improve the absorption
and efficacy of fluorouracil or imiquimod
Tretinoin cream may be used for two weeks prior to fluorouracil treatment to enhance the effect by peeling off
the top layer of skin. This can also reduce the amount of time that fluorouracil needs to be used. N.B. Some patients
with sensitive skin may not tolerate tretinoin. |
Application |
Wash skin with water and dry
Apply thinly with the tip of a finger (using gloves) or a cotton bud – wash hands immediately if not using a
glove
The maximum area of skin that should be treated is 22 cm × 22cm
Hyperkeratotic lesions should be covered with an occlusive dressing to enhance absorption
A topical corticosteroid can be used for symptomatic relief if a severe reaction occurs |
Apply thinly with the tip of a finger, rub into the skin (wash hands after application) and leave on for
eight hours (typically overnight), then wash off any residue with soap and water
One sachet is sufficient to treat a 20 cm × 20 cm area of skin
It is not recommended to apply a topical corticosteroid to treat a reaction (unless severe) as this may affect
the efficacy of imiquimod. If intolerable local adverse effects occur, the cream should be washed off. |
|
If inflammation is extreme, consider less frequent application, a seven-day break from treatment
or, if erosions are present, ceasing treatment |
Dose for actinic keratoses |
Apply once or twice daily, usually for three to four weeks
Monitor weekly for response
Treatment duration may need to be extended (up to eight weeks) if response is slow, e.g. for lesions on the
trunk, lower limbs, hands and forearms, or shortened (one or two weeks) for lesions that are quick to respond,
e.g. flat facial lesions |
Apply two to three times a week for four to six weeks
Assess local response after three weeks and adjust treatment frequency if necessary
Review again after a four week treatment-free interval; treatment can be given for a further six to ten weeks
if the lesion persists |
Dose for superficial BCC |
Apply twice daily, for six to 12 weeks
N.B. Fluorouracil is generally only used to treat small, very superficial BCC, and treatment is associated with
a high rate of recurrence |
Apply to the lesion, including a 1 cm surrounding margin, once daily on five days per week, for six weeks
Review after three weeks (or earlier if the patient reports an extreme reaction) and adjust frequency of application
if necessary depending on response (e.g. reduce to three days per week)
Assess clinical outcome 6 – 12 weeks after treatment has ceased; treatment can be repeated for a further six
to ten weeks if response has been inadequate, provided that there has been at least a four week break since the
first treatment
N.B. best used for BCC <2 cm diameter |
Dose for SCC in situ |
Apply once or twice daily, for three to four weeks
Use an occlusive dressing to increase fluorouracil penetration if tissue reaction is minimal
Assess response; treatment can be continued for a further four weeks, or longer if necessary |
Used off-label for this indication; regimen as for superficial BCC
Apply to the lesion, including a 1 cm surrounding margin, once daily on five days per week, for six weeks
Review after three weeks and adjust frequency of application if necessary depending on response (e.g. reduce
to three days per week)
Assess clinical outcome 6 – 12 weeks after treatment has eased; treatment can be repeated for a further six
to ten weeks if response has been inadequate, provided that there has been at least a four week break since the
first treatment. |
Post-treatment |
Depending on the patients symptoms, petroleum jelly, an emollient or a mild topical corticosteroid can be
used to assist healing. |
|





